Early-life bisphenol A exposure causes neuronal pyroptosis in juvenile and adult male rats through the NF-κB/IL-1β/NLRP3/caspase-1 signaling pathway: exploration of age and dose as effective covariates using an in vivo and in silico modeling approach
- PMID: 38941031
- PMCID: PMC11961519
- DOI: 10.1007/s11010-024-05039-4
Early-life bisphenol A exposure causes neuronal pyroptosis in juvenile and adult male rats through the NF-κB/IL-1β/NLRP3/caspase-1 signaling pathway: exploration of age and dose as effective covariates using an in vivo and in silico modeling approach
Abstract
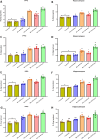
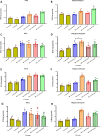
Bisphenol A (BPA), a common endocrine-disrupting chemical, is found in a wide range of home plastics. Early-life BPA exposure has been linked to neurodevelopmental disorders; however, the link between neuroinflammation, pyroptosis, and the development of psychiatric disorders is rarely studied. The current study attempted to investigate the toxic effect of BPA on inflammatory and microglial activation markers, as well as behavioral responses, in the brains of male rats in a dose- and age-dependent manner. Early BPA exposure began on postnatal day (PND) 18 at dosages of 50 and 125 mg/kg/day. We started with a battery of behavioral activities, including open field, elevated plus- and Y-maze tests, performed on young PND 60 rats and adult PND 95 rats. BPA causes anxiogenic-related behaviors, as well as cognitive and memory deficits. The in vivo and in silico analyses revealed for the first time that BPA is a substantial activator of nuclear factor kappa B (NF-κB), interleukin (IL)-1β, -2, -12, cyclooxygenase-2, and the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, with higher beclin-1 and LC3B levels in BPA rats' PFC and hippocampus. Furthermore, BPA increased the co-localization of caspase-1 immunoreactive neurons, as well as unique neurodegenerative histopathological hallmarks. In conclusion, our results support the hypothesis that neuroinflammation and microglial activation are involved with changes in the brain after postnatal BPA exposure and that these alterations may be linked to the development of psychiatric conditions later in life. Collectively, our findings indicate that BPA triggers anxiety-like behaviors and pyroptotic death of nerve cells via the NF-κB/IL-1β/NLRP3/Caspase-1 pathway.
Keywords: Anxiety; Early BPA exposure; Juvenile and adult ages; NF-kB/IL-1β/NLRP3/Caspase-1 pathway; Neuroinflammation; Pyroptosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare there is no conflict of interest. authors declare no competing interests. Ethical approval: This article does not contain any studies with human. Experimental protocols and animal handling procedures were approved by Alexandria University Institutional Animal Care and Use Committee (ALEXU-IACUC), a member of the International Council for Laboratory Animal Science (ICLAS) (Approval number: AU14-22–1003-2–5). Consent to participate: Not applicable. Consent for publication: All authors have agreed to publish this manuscript.
Figures















References
-
- Bakoyiannis I, Kitraki E, Stamatakis A (2021) Endocrine-disrupting chemicals and behaviour: a high risk to take? Best Pract Res Clin Endocrinol Metab 35:e101517 - PubMed
-
- Pironti C, Ricciardi M, Proto A, Bianco PM, Montano L, Motta O (2021) Endocrine-disrupting compounds: an overview on their occurrence in the aquatic environment and human exposure. Water 13:e1347
-
- Abraham A, Chakraborty P (2020) A review on sources and health impacts of bisphenol A. Rev Environ Health 35:201–210 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

