The extracellular matrix protein EMILIN-1 impacts on the microenvironment by hampering gastric cancer development and progression
- PMID: 38941035
- PMCID: PMC11335817
- DOI: 10.1007/s10120-024-01528-z
The extracellular matrix protein EMILIN-1 impacts on the microenvironment by hampering gastric cancer development and progression
Abstract
Background: The contribution of the tumor microenvironment and extracellular matrix to the aggressive biology of Gastric Cancer (GC) has been recently characterized; however, the role of EMILIN-1 in this context is unknown. EMILIN-1 is an essential structural element for the maintenance of lymphatic vessel (LV) integrity and displays anti-proliferative properties as demonstrated in skin and colon cancer. Given the key role of LVs in GC progression, the aim of this study was to investigate the role of EMILIN-1 in GC mouse models.
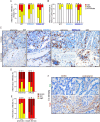
Methods: We used the syngeneic YTN16 cells which were injected subcutaneously and intraperitoneally in genetically modified EMILIN-1 mice. In alternative, carcinogenesis was induced using N-Methyl-N-nitrosourea (MNU). Mouse-derived samples and human biopsies were analyzed by IHC and IF to the possible correlation between EMILIN-1 expression and LV pattern.
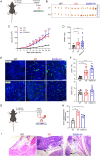
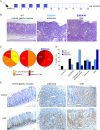
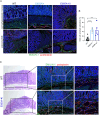
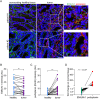
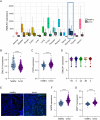
Results: Transgenic mice developed tumors earlier compared to WT animals. 20 days post-injection tumors developed in EMILIN-1 mutant mice were larger and displayed a significant increase of lymphangiogenesis. Treatment of transgenic mice with MNU associated with an increased number of tumors, exacerbated aggressive lesions and higher levels of LV abnormalities. A significant correlation between the levels of EMILIN-1 and podoplanin was detected also in human samples, confirming the results obtained with the pre-clinical models.
Conclusions: This study demonstrates for the first time that loss of EMILIN-1 in GC leads to lymphatic dysfunction and proliferative advantages that sustain tumorigenesis, and assess the use of our animal model as a valuable tool to verify the fate of GC upon loss of EMILIN-1.
Keywords: Extracellular matrix; Gastrointestinal intraepithelial neoplasia; Lymphatic vessels; Mouse models; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

