Integrated Safety and Efficacy Analyses of Phase 3 Trials of a Microbiome Therapeutic for Recurrent CDI
- PMID: 38941068
- PMCID: PMC11416444
- DOI: 10.1007/s40121-024-01007-z
Integrated Safety and Efficacy Analyses of Phase 3 Trials of a Microbiome Therapeutic for Recurrent CDI
Erratum in
-
Correction to: Integrated Safety and Efficacy Analyses of Phase 3 Trials of a Microbiome Therapeutic for Recurrent CDI.Infect Dis Ther. 2024 Oct;13(10):2209-2210. doi: 10.1007/s40121-024-01036-8. Infect Dis Ther. 2024. PMID: 39212853 Free PMC article. No abstract available.
Abstract
Introduction: Recurrent Clostridioides difficile infection (rCDI) often occurs after standard-of-care antibiotics. VOWST oral spores (VOS, previously SER-109), an FDA-approved orally administered microbiome therapeutic, is indicated to prevent rCDI following antibiotics for rCDI.
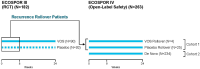
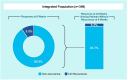
Objective, design, and patients: To evaluate safety and efficacy of VOS from two phase 3 trials, (randomized, placebo-controlled [ECOSPOR III: NCT03183128] and open-label, single arm [ECOSPOR IV: NCT03183141]) of 349 adults with rCDI and prevalent comorbidities.
Methods: VOS or placebo [ECOSPOR III only] (4 capsules once daily for 3 days). Integrated analysis of treatment-emergent adverse events (TEAEs) collected through week 8; serious TEAEs and TEAEs of special interest collected through week 24; and rates of rCDI (toxin-positive diarrhea requiring treatment) evaluated through weeks 8 and 24.
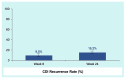
Results: TEAEs were mostly mild or moderate and gastrointestinal. Most common treatment-related TEAEs were flatulence, abdominal pain and distension, fatigue, and diarrhea. There were 11 deaths (3.2%) and 48 patients (13.8%) with serious TEAEs, none treatment-related. The rCDI rate through week 8 was 9.5% (95% CI 6.6-13.0) and remained low through 24 weeks (15.2%; 95% CI 11.6-19.4). Safety and rCDI rates were consistent across subgroups including age, renal impairment/failure, diabetes, and immunocompromise/immunosuppression.
Conclusions: VOS was well tolerated and rates of rCDI remained low through week 24 including in those with comorbidities. These data support the potential benefit of VOS following antibiotics to prevent recurrence in high-risk patients.
Trial registration: ClinicalTrials.gov identifier, NCT03183128 and NCT03183141.
Keywords: Clostridioides difficile infection; Microbiome; Microbiome therapeutics; Recurrent C. difficile infection.
© 2024. The Author(s).
Conflict of interest statement
Colleen Kraft reported serving on the scientific advisory board for Seres Therapeutics and serves as a consultant for Rebiotix/Ferring. Matthew Sims reported serving as an advisory board member for Prenosis, consultant for Applied BioCode, CorMedix, and Venatorx, and also reported serving as a principal investigator or co-investigator for the following companies: AstraZeneca, ContraFect, Crestone, Curetis GmBH, Pfizer, DiaSorin Molecular LLC, Epigenomics Inc, EUROIMMUN US, Finch Therapeutics, Adaptive Phage Genetics Biotest AG—PI, Dompe, Pfizer, Genentech USA Inc, Janssen Research and Development, LLC, Kinevant Sciences GmBH, Leonard-Meron Biosciences, Merck, Prenosis, QIAGEN Sciences LC, Regeneron Pharmaceuticals, Roche, Seres Therapeutics, Shire, and Summit Therapeutics. Christine Lee reported receiving grants from Rebiotix/Ferring, Seres, Merck and Summit Therapeutics to conduct clinical trials. Paul Feuerstadt reported serving as a consultant for Merck and Co. and also reported serving on the speakers bureau and on consulting/advisory boards for the following companies: Seres Therapeutics, Ferring/Rebiotix, and Takeda Pharmaceuticals. Sahil Khanna receives research support from Rebioitx/Ferring, Vedanta, Finch, Seres and Pfizer and serves as a consultant for ProbioTech, Takeda, Niche and Immuron. Colleen Kelly reported serving as a site investigator for Seres Therapeutics and Finch Therapeutics; serving as a clinical advisory board member (unpaid) for Openbiome; as well as serving as a consultant for Sebela Pharmaceuticals. Princy Kumar reported serving as an investigator/receiving research funds from ViiV, Gilead, Merck, and Theratechnologies; serving as a consultant for Viiv, Gilead, and Merck; serving on the Advisory Committee/Board for GSK, Gilead, and Merck; and is a shareholder of Pfizer, GSK, Gilead, and Merck. Brooke Hasson and Lisa von Moltke are employees and shareholders of Seres Therapeutics. Lisa von Moltke is an employee and shareholder of Seres Therapeutics and is also a shareholder and serves on the Board of Directors for Cara Therapeutics. Barbara McGovern, Ananya De, Elaine Wang, Asli Memisoglu, and David Lombardi are all former employees of Seres Therapeutics. Darrell Pardi reported receiving research grants from the following companies: Seres Therapeutics, Vedanta, Finch, Takeda, Applied Molecular Transport and also reported serving as a consultant for: Seres Therapeutics, Vedanta, Immunic Therapeutics, Abbvie, Otsuka, Ferring, Rise Therapeutics, Boehringer Ingelheim, and Summit. Paul Cook is a principal investigator for Gilead, Pfizer, Abbvie and the National Institutes of Health. Louis Korman, Charles Berenson, Mayur Ramesh, Bret Lashner, Alberto Odio, Edward Huang, and Stuart Cohen were study investigators. No other disclosures were reported.
Figures

References
-
- Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemoth. 2013;68(9):1951–61. 10.1093/jac/dkt129. - PubMed
-
- Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–8. 10.1086/525047. - PubMed
-
- Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile Infection. New Engl J Med. 2011;364(5):422–31. 10.1056/nejmoa0910812. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

