The Renin-Angiotensin-Aldosterone System Regulates Sarcoidosis Granulomatous Inflammation
- PMID: 38941161
- PMCID: PMC11351795
- DOI: 10.1164/rccm.202402-0265OC
The Renin-Angiotensin-Aldosterone System Regulates Sarcoidosis Granulomatous Inflammation
Abstract
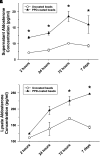
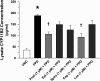
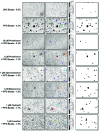
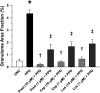
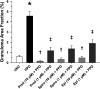
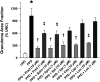
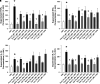
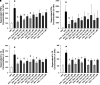
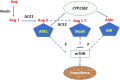
Rationale: Sarcoidosis is a granulomatous disorder of unclear cause notable for abnormal elevation of blood and tissue ACE1 (angiotensin converting enzyme 1) levels and activity. ACE1 regulates the renin-angiotensin-aldosterone system (RAAS), the terminal product of which is aldosterone, which selectively engages mineralocorticoid receptors to promote inflammation. Objectives: We sought to determine whether the RAAS promotes sarcoidosis granuloma formation and related inflammatory responses. Methods: Using an established ex vivo model, we first determined whether aldosterone was produced by sarcoidosis granulomas and verified the presence of CYP11B2, the enzyme required for its production. We then evaluated the effects of selective inhibitors of ACE1 (captopril), angiotensin type 1 receptor (losartan), and mineralocorticoid receptors (spironolactone, eplerenone) on granuloma formation, reflected by computer image analysis-generated granuloma area, and selected cytokines incriminated in sarcoidosis pathogenesis. Measurements and Main Results: Aldosterone was spontaneously produced by sarcoidosis peripheral blood mononuclear cells, and both intra- and extracellular levels steadily increased during granuloma formation. In parallel, peripheral blood mononuclear cells were shown to express more CYP11B2 during granuloma formation. Significant inhibition of sarcoidosis granulomas and related cytokines (TNFα, IL-1β, IFNγ, IL-10) was observed in response to pretreatments with captopril, losartan, spironolactone, or eplerenone, comparable to that of prednisone. Conclusions: The RAAS is intact in sarcoidosis granulomas and contributes significantly to early granuloma formation and to related inflammatory mediator responses, with important implications for clinical management.
Keywords: ACE1; CYP11B2; angiotensin receptor 1; granuloma; mineralocorticoid receptor.
Figures
Comment in
-
Renin-Angiotensin-Aldosterone System: A Potential Source of Biomarkers and Therapeutic Targets for Sarcoidosis.Am J Respir Crit Care Med. 2024 Aug 15;210(4):387-389. doi: 10.1164/rccm.202406-1277ED. Am J Respir Crit Care Med. 2024. PMID: 38990737 Free PMC article. No abstract available.
References
-
- Lieberman J. The specificity and nature of serum-angiotensin-converting enzyme (serum ACE) elevations in sarcoidosis. Ann N Y Acad Sci . 1976;278:488–497. - PubMed
-
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med . 1975;59:365–372. - PubMed
-
- Eklund A, Blaschke E, Danielsson B. Subcellular localization of angiotensin-converting enzyme in the human alveolar macrophage. Scand J Clin Lab Invest . 1987;47:47–54. - PubMed
-
- Silverstein E, Friedland J, Lyons HA, Gourin A. Elevation of angiotensin-converting enzyme in granulomatous lymph nodes and serum in sarcoidosis: clinical and possible pathogenic significance. Ann N Y Acad Sci . 1976;278:498–513. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

