Regulation of blood-brain barrier integrity by Dmp1-expressing astrocytes through mitochondrial transfer
- PMID: 38941455
- PMCID: PMC11212732
- DOI: 10.1126/sciadv.adk2913
Regulation of blood-brain barrier integrity by Dmp1-expressing astrocytes through mitochondrial transfer
Abstract
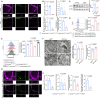
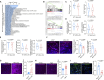
The blood-brain barrier (BBB) acts as the crucial physical filtration structure in the central nervous system. Here, we investigate the role of a specific subset of astrocytes in the regulation of BBB integrity. We showed that Dmp1-expressing astrocytes transfer mitochondria to endothelial cells via their endfeet for maintaining BBB integrity. Deletion of the Mitofusin 2 (Mfn2) gene in Dmp1-expressing astrocytes inhibited the mitochondrial transfer and caused BBB leakage. In addition, the decrease of MFN2 in astrocytes contributes to the age-associated reduction of mitochondrial transfer efficiency and thus compromises the integrity of BBB. Together, we describe a mechanism in which astrocytes regulate BBB integrity through mitochondrial transfer. Our findings provide innnovative insights into the cellular framework that underpins the progressive breakdown of BBB associated with aging and disease.
Figures




References
-
- Begley D. J., Brightman M. W., Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. 61, 39–78 (2003). - PubMed
-
- Abbott N. J., Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 45, 545–552 (2004). - PubMed
-
- Abbott N. J., Rönnbäck L., Hansson E., Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

