Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair
- PMID: 38941462
- PMCID: PMC11212737
- DOI: 10.1126/sciadv.adm8454
Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair
Abstract
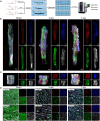
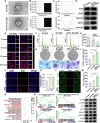
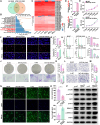
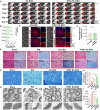
The formation of vascular niche is pivotal during the early stage of peripheral nerve regeneration. Nevertheless, the mechanisms of vascular niche in the regulation of peripheral nerve repair remain unclear. Netrin-1 (NTN1) was found up-regulated in nerve stump after peripheral nerve injury (PNI). Herein, we demonstrated that NTN1-high endothelial cells (NTN1+ECs) were the critical component of vascular niche, fostering angiogenesis, axon regeneration, and repair-related phenotypes. We also found that NTN1+EC-derived exosomes (NTN1 EC-EXO) were involved in the formation of vascular niche as a critical role. Multi-omics analysis further verified that NTN1 EC-EXO carried a low-level expression of let7a-5p and activated key pathways associated with niche formation including focal adhesion, axon guidance, phosphatidylinositol 3-kinase-AKT, and mammalian target of rapamycin signaling pathway. Together, our study suggested that the construction of a pre-regenerative niche induced by NTN1 EC-EXO could establish a beneficial microenvironment for nerve repair and facilitate functional recovery after PNI.
Figures





References
-
- Cattin A. L., Burden J. J., Van Emmenis L., Mackenzie F. E., Hoving J. J., Calavia N. G., Guo Y., McLaughlin M., Rosenberg L. H., Quereda V., Jamecna D., Napoli I., Parrinello S., Enver T., Ruhrberg C., Lloyd A. C., Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162, 1127–1139 (2015). - PMC - PubMed
-
- Liu Y., Cao X., Characteristics and significance of the pre-metastatic niche. Cancer Cell 30, 668–681 (2016). - PubMed
-
- Maruyama K., Takemura N., Martino M. M., Kondo T., Akira S., Netrins as prophylactic targets in skeletal diseases: A double-edged sword? Pharmacol. Res. 122, 46–52 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

