A genome scale transcriptional regulatory model of the human placenta
- PMID: 38941464
- PMCID: PMC11212735
- DOI: 10.1126/sciadv.adf3411
A genome scale transcriptional regulatory model of the human placenta
Abstract
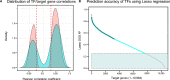
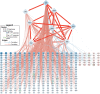
Gene regulation is essential to placental function and fetal development. We built a genome-scale transcriptional regulatory network (TRN) of the human placenta using digital genomic footprinting and transcriptomic data. We integrated 475 transcriptomes and 12 DNase hypersensitivity datasets from placental samples to globally and quantitatively map transcription factor (TF)-target gene interactions. In an independent dataset, the TRN model predicted target gene expression with an out-of-sample R2 greater than 0.25 for 73% of target genes. We performed siRNA knockdowns of four TFs and achieved concordance between the predicted gene targets in our TRN and differences in expression of knockdowns with an accuracy of >0.7 for three of the four TFs. Our final model contained 113,158 interactions across 391 TFs and 7712 target genes and is publicly available. We identified 29 TFs which were significantly enriched as regulators for genes previously associated with preterm birth, and eight of these TFs were decreased in preterm placentas.
Figures





References
-
- Burton G. J., Jauniaux E., What is the placenta? Am. J. Obstet. Gynecol. 213, S6–S8 (2015). - PubMed
-
- S. Mesiano, CHAPTER 11 - The endocrinology of human pregnancy and fetoplacental neuroendocrine development A2 - Strauss, Jerome F. in Yen and Jaffe’s Reproductive Endocrinology (Sixth Edition), R. L. Barbieri, Ed. (W.B. Saunders, 2009; https://sciencedirect.com/science/article/pii/B9781416049074000115), pp. 249–281.
-
- Y. Wang, S. Zhao, Vascular Biology of the Placenta (Morgan & Claypool Life Sciences, 2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

