Remote Functionalization by Pd-Catalyzed Isomerization of Alkynyl Alcohols
- PMID: 38941513
- PMCID: PMC11240579
- DOI: 10.1021/jacs.4c05136
Remote Functionalization by Pd-Catalyzed Isomerization of Alkynyl Alcohols
Abstract
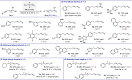
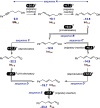
In recent years, progress has been made in the development of catalytic methods that allow remote functionalizations based on alkene isomerization. In contrast, protocols based on alkyne isomerization are comparatively rare. Herein, we report a general Pd-catalyzed long-range isomerization of alkynyl alcohols. Starting from aryl-, heteroaryl-, or alkyl-substituted precursors, the optimized system provides access preferentially to the thermodynamically more stable α,β-unsaturated aldehydes and is compatible with potentially sensitive functional groups. We showed that the migration of both π-components of the carbon-carbon triple bond can be sustained over several methylene units. Computational investigations served to shed light on the key elementary steps responsible for the reactivity and selectivity. These include an unorthodox phosphine-assisted deprotonation rather than a more conventional β-hydride elimination in the final tautomerization event.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Larionov E.; Li H.; Mazet C. Well-Defined Transition Metal Hydrides in Catalytic Isomerizations. Chem. Commun. 2014, 50, 9816.10.1039/C4CC02399D. - DOI - PubMed
- Vasseur A.; Bruffaerts J.; Marek I. Remote Functionalization through Alkene Isomerization. Nat. Chem. 2016, 8, 209.10.1038/nchem.2445. - DOI - PubMed
- Sommer H.; Julia-Hernández F.; Martin R.; Marek I. Walking Metals for Remote Functionalization. ACS Cent. Sci. 2018, 4, 153.10.1021/acscentsci.8b00005. - DOI - PMC - PubMed
- Kochi T.; Kanno S.; Kakiuchi F. Nondissociative Chain Walking as a Strategy in Catalytic Organic Synthesis. Tetrahedron Lett. 2019, 60, 15093810.1016/j.tetlet.2019.07.029. - DOI
- Massad I.; Marek I. Alkene Isomerization through Allylmetals as a Strategic Tool in Stereoselective Synthesis. ACS Catal. 2020, 10, 5793.10.1021/acscatal.0c01174. - DOI
- Janssen-Müller D.; Sahoo B.; Sun S.-Z.; Martin R. Tackling Remote sp3 C–H Functionalization via Ni–Catalyzed “chain–walking” Reactions. Isr. J. Chem. 2020, 60, 195.10.1002/ijch.201900072. - DOI
- Fiorito D.; Scaringi S.; Mazet C. Transition metal–catalyzed alkene isomerization as an enabling technology in tandem, sequential and domino processes. Chem. Soc. Rev. 2021, 50, 1391.10.1039/D0CS00449A. - DOI - PubMed
- Ghosh S.; Patel S.; Chatterjee I. Chain-walking reactions of transition metals for remote C–H bond functionalization of olefinic substrates. Chem. Commun. 2021, 57, 11110.10.1039/D1CC04370F. - DOI - PubMed
-
-
For a review, see:
- Scaringi S.; Mazet C. Transition Metal-Catalyzed (Remote) Deconjugative Isomerization of α,β-Unsaturated Carbonyls. Tetrahedron Lett. 2022, 96, 15375610.1016/j.tetlet.2022.153756. - DOI
-
For selected examples using transition metal catalysts, see:
- Lin L.; Romano C.; Mazet C. Palladium-Catalyzed Long-Range Deconjugative Isomerization of Highly Substituted α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2016, 138, 10344.10.1021/jacs.6b06390. - DOI - PubMed
- Borah A. J.; Shi Z.-Z. Rhodium-Catalyzed, Remote Terminal Hydroarylation of Activated Olefins through a Long-Range Deconjugative Isomerization. J. Am. Chem. Soc. 2018, 140, 6062.10.1021/jacs.8b03560. - DOI - PubMed
- Hanna S.; Butcher T. W.; Hartwig J. F. Contra-thermodynamic Olefin Isomerization by Chain-Walking Hydrofunctionalization and Formal Retro-hydrofunctionalization. Org. Lett. 2019, 21, 7129.10.1021/acs.orglett.9b02695. - DOI - PubMed
- Li C.; Zhang K.; Zhao W. ACS Catal. 2024, 14, 5458.10.1021/acscatal.3c06194. - DOI
-
-
- Avocetien K.; Li Y.; O’Doherty G. A.. The Alkyne Zipper Reaction in Asymmetric Synthesis; Barry M.; Trost C.-J. L., Eds.; Wiley and Sons: Weinheim, Germany, 2014.
-
- Danilkina N. A.; Vasileva A. A.; Balova I. A. A.E. Favorskii’s scientific legacy in modern organic chemistry: prototropic acetylene – allene isomerization and the acetylene zipper reaction. Russ. Chem. Rev. 2020, 89, 125.10.1070/RCR4902. - DOI
- Sørskår Å. M.; Stenstrøm H. Ø. K.; Stenstrøm Y.; Antonsen S. G.; et al. The Alkyne Zipper Reaction: A Useful Tool in Synthetic Chemistry. Reactions 2023, 4, 26.10.3390/reactions4010002. - DOI
-
-
For selected examples of alkyne zipper in synthesis, see:
- Kirkham J. E. D.; Courtney T. D. L.; Lee V.; Baldwin J. E. Asymmetric synthesis of cytotoxic sponge metabolites R-strongylodiols A and B. Tetrahedron Lett. 2004, 45, 5645.10.1016/j.tetlet.2004.05.122. - DOI
- Hoye R. C.; Anderson G. L.; Brown S. G.; Schultz E. E. Total Synthesis of Clathculins A and B. J. Org. Chem. 2010, 75, 7400.10.1021/jo100971j. - DOI - PubMed
- Das S.; Kuilya T. K.; Goswami R. K. Asymmetric Total Synthesis of Bioactive Natural Lipid Mycalol. J. Org. Chem. 2015, 80, 6467.10.1021/acs.joc.5b00972. - DOI - PubMed
- Avocetien K. F.; Li J. J.; Liu X.; Wang Y.; Xing Y.; O’Doherty G. A. De Novo Asymmetric Synthesis of Phoracantholide. Org. Lett. 2016, 18, 4970.10.1021/acs.orglett.6b02432. - DOI - PubMed
- Xu F.; Zhong Y. L.; Li H. M.; Qi J.; Desmond R.; Song Z. G. J.; Park J.; Wang T.; Truppo M.; Humphrey G. R.; Ruck R. T. Asymmetric Synthesis of Functionalized Trans-Cyclopropoxy Building Block for Grazoprevir. Org. Lett. 2017, 19, 5880.10.1021/acs.orglett.7b02867. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

