LMWH prevents thromboinflammation in the placenta via HBEGF-AKT signaling
- PMID: 38941535
- PMCID: PMC11457404
- DOI: 10.1182/bloodadvances.2023011895
LMWH prevents thromboinflammation in the placenta via HBEGF-AKT signaling
Abstract
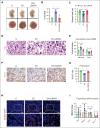
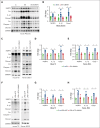
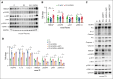
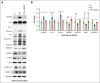
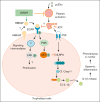
Low molecular weight heparins (LMWH) are used to prevent or treat thromboembolic events during pregnancy. Although studies suggest an overall protective effect of LMWH in preeclampsia (PE), their use in PE remains controversial. LMWH may convey beneficial effects in PE independent of their anticoagulant activity, possibly by inhibiting inflammation. Here, we evaluated whether LMWH inhibit placental thromboinflammation and trophoblast NLRP3 inflammasome activation. Using an established procoagulant extracellular vesicle-induced and platelet-dependent PE-like mouse model, we show that LMWH reduces pregnancy loss and trophoblast inflammasome activation, restores altered trophoblast differentiation, and improves trophoblast proliferation in vivo and in vitro. Moreover, LMWH inhibits platelet-independent trophoblast NLRP3 (NLR family pyrin domain containing 3) inflammasome activation. Mechanistically, LMWH activates via heparin-binding epidermal growth factor (HBEGF) signaling the PI3-kinase-AKT pathway in trophoblasts, thus preventing inflammasome activation. In human PE placental explants, inflammasome activation and PI3-kinase-AKT signaling events were reduced with LMWH treatment compared with those without LMWH treatment. Thus, LMWH inhibits sterile inflammation via the HBEGF signaling pathway in trophoblasts and ameliorates PE-associated complications. These findings suggest that drugs targeting the inflammasome may be evaluated in PE and identify a signaling mechanism through which LMWH ameliorates PE, thus providing a rationale for the use of LMWH in PE.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–538. - PubMed
-
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975. - PubMed
-
- Lockwood CJ. Pregnancy-associated changes in the hemostatic system. Clin Obstet Gynecol. 2006;49(4):836–843. - PubMed
-
- Kohli S, Ranjan S, Hoffmann J, et al. Maternal extracellular vesicles and platelets promote preeclampsia through inflammasome activation in embryonic trophoblast. Blood. 2016;128(17):2153–2164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

