Intensive induction chemotherapy vs hypomethylating agents in combination with venetoclax in NPM1-mutant AML
- PMID: 38941537
- PMCID: PMC11416634
- DOI: 10.1182/bloodadvances.2024012858
Intensive induction chemotherapy vs hypomethylating agents in combination with venetoclax in NPM1-mutant AML
Abstract
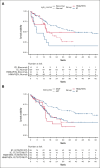
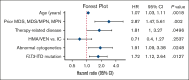
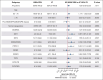
Although intensive induction chemotherapy (IC) remains the standard of care for younger patients with acute myeloid leukemia (AML), hypomethylating agents + venetoclax (HMA/VEN) can lead to durable remission among older patients with nucleophosmin 1 (NPM1) mutations. Whether IC or HMA/VEN is superior in patients aged ≥60 years with NPM1-mutant AML is unknown. We performed an international, multicenter retrospective cohort study of 221 patients (147 IC and 74 HMA/VEN) with previously untreated NPM1-mutant AML. Composite complete remission (cCR) (defined as CR + CR with incomplete count recovery) rate was similar for IC and HMA/VEN (cCR, 85% vs 74%; P = .067). Although overall survival (OS) was favorable with IC in unselected patients compared with HMA/VEN (24-month OS, 59% [95% confidence interval (CI), 52-69%] vs 38% [95% CI, 27-55%]; P = .013), it was not statistically different among patients aged 60-75 years (60% [95% CI, 52-70%] vs 44% [95% CI, 29-66%]; P = .069) and patients who received an allogeneic stem cell transplant (70% [95% CI, 58-85%] vs 66% [95% CI, 44-100%]; P = .56). Subgroup analyses suggested that patients with normal cytogenetics (24-month OS, 65% [95% CI, 56-74%] with IC vs 40% [95% CI, 26-60%] with HMA/VEN; P = .009) and without FLT3 internal tandem duplication mutations might benefit from IC compared with HMA/VEN (24-month OS, 68% [95% CI, 59-79%] vs 43% [95% CI, 29-63%]; P = .008). In multivariable analysis, OS was not statistically different between patients treated with IC and HMA/VEN (hazard ratio for death with HMA/VEN vs IC, 0.71; 95% CI, 0.40-1.27; P = .25).
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.M. Shallis provided consultancy for Bristol Myers Squibb (BMS), Curio Science, Gilead Sciences, Servier, and Rigel. A.M.Z. received research funding (institutional) from Celgene/BMS, AbbVie, Astex, Pfizer, MedImmune/AstraZeneca, Boehringer Ingelheim, Cardiff Oncology, Incyte, Takeda Pharmaceuticals, Novartis, Aprea Therapeutics, and ADC Therapeutics; participated in advisory boards, provided consultancy for, and received honoraria from AbbVie, Otsuka Pharmaceutical, Pfizer, Celgene/BMS, Jazz Pharmaceuticals, Incyte, Agios Pharmaceuticals, Boehringer Ingelheim, Novartis, Acceleron Pharma, Astellas Pharma, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Cardiff Oncology, Takeda Pharmaceuticals, Ionis, Amgen, Janssen, Epizyme, Syndax, Gilead, Kura, Chiesi, ALX Oncology, BioCryst, Notable Labs, Orum, and Tyme; and served on clinical trial committees for Novartis, AbbVie, Gilead, BioCryst, AbbVie, ALX Oncology, Geron, and Celgene/BMS. A.D.G. participated in advisory boards and provided consultancy for AbbVie, Astellas Pharma, Daiichi Sankyo, Genentech, BMS, and Molecular Partners; received honoraria from DAVA Oncology; received research funding from AbbVie, Aprea, Aptose Biosciences, AROG Pharmaceuticals, Celularity, Kura, Pfizer, and Prelude; and served on safety monitoring committees for AbbVie and Kura. E.M.S. received research funding from Bayer, Syndax, Daiichi Sankyo, Celgene Pharmaceuticals, and Novartis; served as a consultant for Amgen, AbbVie, Seattle Genetics, Biotheryx, Syndax, Astellas Pharmaceutical, Agios Pharmaceuticals, Genentech, Daiichi Sankyo, Celgene Pharmaceuticals, and Novartis; was a member of the board of directors or advisory committee for PTC Therapeutics, Syros, Astellas Pharmaceutical, Agios Pharmaceuticals, Genentech, Daiichi Sankyo, Celgene Pharmaceuticals, and Novartis; and is a current equity holder in privately held Auron Therapeutics. R.C.L. provided consultancy for Takeda Pharmaceuticals, bluebird bio, Qiagen, Sarepta Therapeutics, Verve Therapeutics, Jazz Pharmaceuticals, and Vertex Pharmaceuticals. E.C.C. received research funding from AbbVie and provided consultancy for Rigel Pharmaceuticals. A.S. declares stock ownership in Sanofi. D.J.D. received honoraria from Amgen, Autolus, Blueprint, Gilead, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda Pharmaceuticals, and research funding from AbbVie, Novartis, Bluprint, and Glycomimetics. D.S.N. declares equity ownership in Madrigal Pharmaceuticals. R.M. Stone provided consultancy for AbbVie, CTI Biopharma, GlaxoSmithKline, Hermavant, Ligand Pharma, Lava Therapeutics, Amgen, AvenCell, BerGenBio, Celularity, Jazz Pharmaceuticals, Kura One, and Rigel, and participated in drug and safety monitoring boards for Aptevo, Epizyme, Takeda Pharmaceuticals, and Syntrix. S.G. declares a consulting or advisory role with AbbVie, Astellas, BMS/Celgene, Jazz Pharmaceuticals, and Servier, and received travel grants from Gilead. B.B. served on advisory boards for BMS, Rigel, and Oncovalent Therapeutics. M.S. served on advisory boards for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, Rigel, BMS, and Taiho; consulted for Boston Consulting and Dedham Group; and participated in CME activities for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. The remaining authors declare no competing financial interests.
Figures


References
-
- Falini B. NPM1-mutated acute myeloid leukemia: new pathogenetic and therapeutic insights and open questions. Am J Hematol. 2023;98(9):1452–1464. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 2022;140(12):1345–1377. - PubMed
-
- Erba HP, Montesinos P, Kim H-J, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10388):1571–1583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

