Inflammasome activation in patients with Kaposi sarcoma herpesvirus-associated diseases
- PMID: 38941593
- PMCID: PMC11474434
- DOI: 10.1182/blood.2024024144
Inflammasome activation in patients with Kaposi sarcoma herpesvirus-associated diseases
Abstract
Kaposi sarcoma herpesvirus (KSHV)-associated diseases include Kaposi sarcoma (KS), primary effusion lymphoma (PEL), KSHV-associated multicentric Castleman disease (MCD), and KS inflammatory cytokine syndrome (KICS). PEL, MCD, and KICS are associated with elevated circulating inflammatory cytokines. However, activation of the inflammasome, which generates interleukin-1β (IL-1β) and IL-18 via active caspase-1/4/5, has not been evaluated in patients with KSHV-associated diseases (KADs). Herein we report that patients with HIV and ≥1 KAD present with higher plasma levels of IL-18 and increased caspase-1/4/5 activity in circulating monocytes compared with HIV-negative healthy volunteers (HVs) or people with HIV (PWH) without KAD. Within KAD subtypes, KICS and MCD shared enhanced caspase-1/4/5 activity and IL-18 production compared with HVs and PWH, whereas patients with PEL showed remarkably high levels of inflammasome complex formation (known as apoptosis-associated speck-like protein containing a caspase recruitment domain). Moreover, caspase-1/4/5 activity and IL-18 plasma levels correlated with KSHV viral load, indicating KSHV-driven inflammasome activation in KAD. Accordingly, factors released by cells latently infected with KSHV triggered inflammasome activation and cytokine production in bystander monocytes in vitro. Finally, both supervised and unsupervised analyses with inflammasome measurements and other inflammatory biomarkers demonstrate a unique inflammatory profile in patients with PEL, MCD, and KICS as compared with KS. Our data indicate that detrimental inflammation in patients with KAD is at least partially driven by KSHV-induced inflammasome activation in monocytes, thus offering novel approaches to diagnose and treat these complex disorders. These trials were registered at www.ClinicalTrials.gov as #NCT01419561, NCT00092222, NCT00006518, and NCT02147405.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: D.W. and A.R. are employed by Leidos Biomedical Research, Inc. R.R., K.L., and R.Y. report receiving research support from Celgene/Bristol Myers Squibb, CTI BioPharma (a Sobi A.B. company), PBS Biotech, and Janssen Pharmaceuticals through Cooperative Research and Development Agreements (CRADAs) with the National Cancer Institute (NCI). R.R., K.L., and R.Y. report receiving drugs for a clinical trial from Merck, EMD Serono, and Eli Lilly through CRADAs with the NCI. I.S., R.Y., and D.W. are coinventors on US patent 10,001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds and uses of biomarkers.” An immediate family member of R.Y. is a coinventor on patents or patent applications related to internalization of target receptors, epigenetic analysis, and ephrin tyrosine kinase inhibitors. All rights, title, and interest to these patents have been assigned to the US Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing financial interests.
Figures

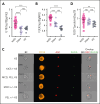



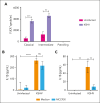
Comment in
-
Is inflammation key in Kaposi sarcoma?Blood. 2024 Oct 3;144(14):1464-1465. doi: 10.1182/blood.2024025829. Blood. 2024. PMID: 39361305 Free PMC article. No abstract available.
References
-
- Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(22):2145. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

