Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain
- PMID: 38942014
- PMCID: PMC12344723
- DOI: 10.1016/j.cell.2024.05.058
Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain
Abstract
Neuroimmune interactions mediate intercellular communication and underlie critical brain functions. Microglia, CNS-resident macrophages, modulate the brain through direct physical interactions and the secretion of molecules. One such secreted factor, the complement protein C1q, contributes to complement-mediated synapse elimination in both developmental and disease models, yet brain C1q protein levels increase significantly throughout aging. Here, we report that C1q interacts with neuronal ribonucleoprotein (RNP) complexes in an age-dependent manner. Purified C1q protein undergoes RNA-dependent liquid-liquid phase separation (LLPS) in vitro, and the interaction of C1q with neuronal RNP complexes in vivo is dependent on RNA and endocytosis. Mice lacking C1q have age-specific alterations in neuronal protein synthesis in vivo and impaired fear memory extinction. Together, our findings reveal a biophysical property of C1q that underlies RNA- and age-dependent neuronal interactions and demonstrate a role of C1q in critical intracellular neuronal processes.
Keywords: C1q; RNA granule; RNA-binding protein; complement; liquid-liquid phase separation; microglia; neuroimmune; neuronal translation; polysome; ribonucleoprotein complex.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.S. serves on the Scientific Advisory Board of Annexon Biosciences and is a minor shareholder of Annexon.
Figures

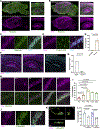

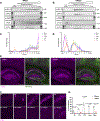
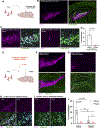
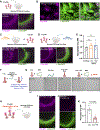
References
-
- Schafer DP., Lehrman EK., Kautzman AG., Koyama R., Mardinly AR., Yamasaki R., Ransohoff RM., Greenberg ME., Barres BA., and Stevens B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. 10.1016/j.neuron.2012.03.026. - DOI - PMC - PubMed
-
- Dejanovic B, Wu T, Tsai M-C, Graykowski D, Gandham VD, Rose CM, Bakalarski CE, Ngu H, Wang Y, Pandey S, et al. (2022). Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nature Aging 2, 837–850. 10.1038/s43587-022-00281-1. - DOI - PMC - PubMed
-
- Werneburg S, Jung J, Kunjamma RB, Ha SK, Luciano NJ, Willis CM, Gao G, Biscola NP, Havton LA, Crocker SJ, et al. (2020). Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity 52, 167–182.e167. 10.1016/j.immuni.2019.12.004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

