Subclinical leaflet thrombus in patients with severe aortic stenosis and atrial fibrillation -ENRICH-AF TAVI study
- PMID: 38942790
- PMCID: PMC11213935
- DOI: 10.1038/s41598-024-65600-5
Subclinical leaflet thrombus in patients with severe aortic stenosis and atrial fibrillation -ENRICH-AF TAVI study
Abstract
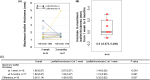
Subclinical leaflet thrombosis (SLT) can be one of the causes of transcatheter heart valve (THV) failure after transcatheter aortic valve implantation (TAVI). We sought to clarify the formation process of SLT and thrombogenicity during the perioperative period of TAVI. This multicenter, prospective, single-arm interventional study enrolled 26 patients treated with edoxaban for atrial fibrillation and who underwent TAVI for severe aortic stenosis between September 2018 and September 2022. We investigated changes in maximal leaflet thickness detected by contrast-enhanced computed tomography between 1 week and 3 months after TAVI in 18 patients and measured the thrombogenicity by Total Thrombus-formation Analysis System (T-TAS) and flow stagnation volume by computational fluid dynamics (CFD) (n = 11). SLT was observed in 16.7% (3/18) at 1 week, but decreased to 5.9% (1/17) at 3 months after TAVI. Patients with SLT at 1 week had a significantly decreased maximal leaflet thickness compared to those without SLT. Thrombogenicity assessed by T-TAS decreased markedly at 1 week and tended to increase at 3 months. The stagnation volume assessed by CFD was positively associated with a higher maximum leaflet thickness. This study showed the course of leaflet thrombus formation and visualization of stagnation in neo-sinus of THV in the acute phase after TAVI.
Keywords: CFD; DOAC; Leaflet thrombosis; T-TAS; TAVI.
© 2024. The Author(s).
Conflict of interest statement
Dr. Kaikita reports remuneration for lectures from Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Novartis Pharma AG, and Otsuka Pharmaceutical Co., Ltd.; has received trust research/joint research funds from Bayer Yakuhin, Ltd., and Daiichi Sankyo Co., Ltd.; and has received scholarship funds from Abbott Medical Co., Ltd.. Dr. Tsujita received significant research grant from AMI Co., Ltd., Bayer Yakuhin, Ltd., Bristol-Myers K.K., EA Pharma Co. Ltd., Mochida Pharmaceutical Co., Ltd., and scholarship fund from AMI Co., Ltd., Bayer Yakuhin, Ltd., Boehringer Ingelheim Japan, Chugai Pharmaceutical Co, Ltd., Daiichi Sankyo Co., Ltd., Edwards Lifesciences Corporation, Johnson & Johnson K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and honoraria from Amgen K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co. Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., and belongs to the endowed departments donated by Abbott Japan Co., Ltd., Boston Scientific Japan K.K., Fides-one, Inc., GM Medical Co., Ltd., ITI Co., Ltd., Kaneka Medix Co., Ltd., NIPRO CORPORATION, TERUMO Co, Ltd., Abbott Medical Co., Ltd., Cardinal Heaith Japan, Fukuda Denshi Co., Ltd., Japan Lifeline Co., Ltd., Medical Appliance Co., Ltd., Medtoronic Japan Co., Ltd. The remaining authors have nothing to disclose.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

