The chemical language of protein glycation
- PMID: 38942948
- PMCID: PMC12020258
- DOI: 10.1038/s41589-024-01644-y
The chemical language of protein glycation
Abstract
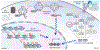
Glycation is a non-enzymatic post-translational modification (PTM) that is correlated with many diseases, including diabetes, cancer and age-related disorders. Although recent work points to the importance of glycation as a functional PTM, it remains an open question whether glycation has a causal role in cellular signaling and/or disease development. In this Review, we contextualize glycation as a specific mechanism of carbon stress and consolidate what is known about advanced glycation end-product (AGE) structures and mechanisms. We highlight the current understanding of glycation as a PTM, focusing on mechanisms for installing, removing or recognizing AGEs. Finally, we discuss challenges that have hampered a more complete understanding of the biological consequences of glycation. The development of tools for predicting, modulating, mimicking or capturing glycation will be essential for interpreting a post-translational glycation network. Therefore, continued insights into the chemistry of glycation will be necessary to advance understanding of glycation biology.
© 2024. Springer Nature America, Inc.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Li Z; Li S; Luo M; Jhong J-H; Li W; Yao L; Pang Y; Wang Z; Wang R; Ma R; Yu J; Huang Y; Zhu X; Cheng Q; Feng H; Zhang J; Wang C; Hsu JB-K; Chang W-C; Wei F-X; Huang H-D; Lee T-Y dbPTM in 2022: An Updated Database for Exploring Regulatory Networks and Functional Associations of Protein Post-Translational Modifications. Nucleic Acids Res. 2022, 50 (D1), D471–D479. 10.1093/nar/gkab1017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

