Safety and Effectiveness of Irreversible Electroporation in Lymph Node Metastases
- PMID: 38943032
- PMCID: PMC11303484
- DOI: 10.1007/s00270-024-03795-w
Safety and Effectiveness of Irreversible Electroporation in Lymph Node Metastases
Abstract
Purpose: Demonstrating the safety and efficacy of percutaneous irreversible electroporation (IRE) for the treatment of lymph node metastases.
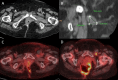
Materials and methods: An IRB-approved, single-center retrospective review was performed on patients with lymph node metastases gastrointestinal, and genitourinary primary cancers. Primary objective safety was evaluated by assessing complications graded according to the Clavien-Dindo Classification, and efficacy was determined by tumor response on follow-up imaging and local progression-free survival (LPFS). Secondary outcome measures were technical success (complete ablation with an adequate ablative margin > 5 mm), length of hospital stay and distant progression-free survival (DPFS).
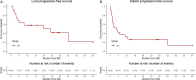
Results: Nineteen patients underwent percutaneous IRE between June 2018 and February 2023 for lymph node metastases, close to critical structures, such as vasculature, bowel, or nerves. The technical success was achieved in all cases. Complications occurred in four patients (21.1%), including two self-limiting grade 1 hematomas, a grade 1 abdominal pain, and grade 2 nerve pain treated with medication. Seventeen patients were hospitalized overnight, one patient stayed two nights and another patient stayed fourteen nights. Median follow-up was 25.5 months. Median time to local progression was 24.1 months (95% CI: 0-52.8) with 1-, 2-, and 5-year LPFS of 57.9%, 57.9% and 20.7%, respectively. Median time to distant progression was 4.3 months (95% CI: 0.3-8.3) with 1-, 2-, and 5-year DPFS of 31.6%, 13.2% and 13.2%, respectively.
Conclusion: IRE is a safe and effective minimally-invasive treatment for lymph node metastases in locations, where temperature dependent ablation may be contraindicated. Care should be taken when employing IRE near nerves.
Keywords: Irreversible electroporation (IRE); Lymph node metastases; Tumor response.
© 2024. The Author(s).
Conflict of interest statement
The other authors declare that they have no conflict of interest.
Figures
Similar articles
-
Minimally Invasive Image-Guided Percutaneous Irreversible Electroporation of Adrenal Metastases.Cardiovasc Intervent Radiol. 2025 Jan;48(1):77-83. doi: 10.1007/s00270-024-03893-9. Epub 2024 Nov 25. Cardiovasc Intervent Radiol. 2025. PMID: 39586931
-
Feasibility and safety of irreversible electroporation (IRE) in patients with small renal masses: Results of a prospective study.Urol Oncol. 2019 Mar;37(3):183.e1-183.e8. doi: 10.1016/j.urolonc.2018.11.008. Epub 2018 Nov 30. Urol Oncol. 2019. PMID: 30509869 Clinical Trial.
-
Safety and Efficacy of Irreversible Electroporation for the Treatment of Hepatocellular Carcinoma Not Amenable to Thermal Ablation Techniques: A Retrospective Single-Center Case Series.Radiology. 2017 Sep;284(3):877-886. doi: 10.1148/radiol.2017161413. Epub 2017 Apr 28. Radiology. 2017. PMID: 28453431
-
Is irreversible electroporation safe and effective in the treatment of hepatobiliary and pancreatic cancers?Hepatobiliary Pancreat Dis Int. 2019 Apr;18(2):117-124. doi: 10.1016/j.hbpd.2019.01.001. Epub 2019 Jan 4. Hepatobiliary Pancreat Dis Int. 2019. PMID: 30655073 Review.
-
Irreversible Electroporation for the Ablation of Prostate Cancer.Curr Urol Rep. 2019 Sep 2;20(10):63. doi: 10.1007/s11934-019-0929-x. Curr Urol Rep. 2019. PMID: 31478109 Review.
Cited by
-
Interventional oncology and immunotherapy: current status and future perspectives.Front Immunol. 2025 Apr 8;16:1541105. doi: 10.3389/fimmu.2025.1541105. eCollection 2025. Front Immunol. 2025. PMID: 40264767 Free PMC article. Review.
References
-
- Jingu K, Ariga H, Nemoto K, Narazaki K, Umezawa R, Takeda K, et al. Long term results of radiochemotherapy for solitary lymph node metastasis after curative resection of esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:172–7. 10.1016/j.ijrobp.2011.06.1978. 10.1016/j.ijrobp.2011.06.1978 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

