Identification of T2W hypointense ring as a novel noninvasive indicator for glioma grade and IDH genotype
- PMID: 38943156
- PMCID: PMC11212435
- DOI: 10.1186/s40644-024-00726-3
Identification of T2W hypointense ring as a novel noninvasive indicator for glioma grade and IDH genotype
Abstract
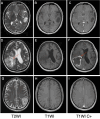
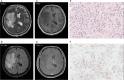
Background: This study aimed to evaluate the T2W hypointense ring and T2-FLAIR mismatch signs in gliomas and use these signs to construct prediction models for glioma grading and isocitrate dehydrogenase (IDH) mutation status.
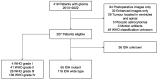
Methods: Two independent radiologists retrospectively evaluated 207 glioma patients to assess the presence of T2W hypointense ring and T2-FLAIR mismatch signs. The inter-rater reliability was calculated using the Cohen's kappa statistic. Two logistic regression models were constructed to differentiate glioma grade and predict IDH genotype noninvasively, respectively. Receiver operating characteristic (ROC) analysis was used to evaluate the developed models.
Results: Of the 207 patients enrolled (119 males and 88 females, mean age 51.6 ± 14.8 years), 45 cases were low-grade gliomas (LGGs), 162 were high-grade gliomas (HGGs), 55 patients had IDH mutations, and 116 were IDH wild-type. The number of T2W hypointense ring signs was higher in HGGs compared to LGGs (p < 0.001) and higher in the IDH wild-type group than in the IDH mutant group (p < 0.001). There were also significant differences in T2-FLAIR mismatch signs between HGGs and LGGs, as well as between IDH mutant and wild-type groups (p < 0.001). Two predictive models incorporating T2W hypointense ring, absence of T2-FLAIR mismatch, and age were constructed. The area under the ROC curve (AUROC) was 0.940 for predicting HGGs (95% CI = 0.907-0.972) and 0.830 for differentiating IDH wild-type (95% CI = 0.757-0.904).
Conclusions: The combination of T2W hypointense ring, absence of T2-FLAIR mismatch, and age demonstrate good predictive capability for HGGs and IDH wild-type. These findings suggest that MRI can be used noninvasively to predict glioma grading and IDH mutation status, which may have important implications for patient management and treatment planning.
Keywords: Glioma; Isocitrate dehydrogenase; Magnetic resonance imaging; T2-FLAIR mismatch; T2W hypointense ring.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- SHDC2022CRT025/Clinical Research and Cultivation Project of Shanghai ShenKang Hospital Development Center
- SKLY2022CRT402/the Joint Research Development Project be-tween Shenkang and United Imaging on Clinical Research and Translation
- ZDXK2209/National Key Clinical Specialty Discipline Construction Program of China
LinkOut - more resources
Full Text Sources
Medical

