Effect of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes treated with an implantable cardioverter-defibrillator: the EMPA-ICD trial
- PMID: 38943159
- PMCID: PMC11214255
- DOI: 10.1186/s12933-024-02309-9
Effect of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes treated with an implantable cardioverter-defibrillator: the EMPA-ICD trial
Abstract
Background: Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce the risk of hospitalization for heart failure and cardiovascular death with type 2 diabetes; however, their effect on arrhythmias is unclear. The purpose of this study was to investigate the effects of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes.
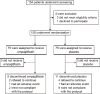
Methods: A total of 150 patients with type 2 diabetes who were treated with an implantable cardioverter-defibrillator or cardiac resynchronization therapy defibrillator (ICD/CRT-D) were randomized to once-daily empagliflozin or placebo for 24 weeks. The primary endpoint was the change in the number of ventricular arrhythmias from the 24 weeks before to the 24 weeks during treatment. Secondary endpoints included the change in the number of appropriate device discharges and other values.
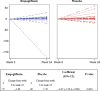
Results: In the empagliflozin group, the number of ventricular arrhythmias recorded by ICD/CRT-D decreased by 1.69 during treatment compared to before treatment, while in the placebo group, the number increased by 1.79. The coefficient for the between-group difference was - 1.07 (95% confidence interval [CI] - 1.29 to - 0.86; P < 0.001). The change in the number of appropriate device discharges during and before treatment was 0.06 in the empagliflozin group and 0.27 in the placebo group, with no significant difference between the groups (P = 0.204). Empagliflozin was associated with an increase in blood ketones and hematocrit and a decrease in blood brain natriuretic peptide and body weight.
Conclusions: In patients with type 2 diabetes treated with ICD/CRT-D, empagliflozin reduces the number of ventricular arrhythmias compared with placebo. Trial registration jRCTs031180120.
Keywords: Empagliflozin; Sodium-glucose cotransporter 2; Type 2 diabetes; Ventricular arrhythmia.
© 2024. The Author(s).
Conflict of interest statement
S.F. received funding support of present manuscript from Nippon Boehringer Ingelheim Co. Ltd, Eli Lilly and Company. Y.N. received grant and speaking honoraria from Nippon Boehringer Ingelheim. K.K. received honoraria for lectures from Medtronic, Boehringer-Ingelheim. Y.K. received speaking honoraria from Biotronik Japan, Boston Scientific Japan, Daiichi-Sankyo, Bayer Yakuhin, Abbott Medical Japan, Japan Lifeline, and received research grant from Daiichi-Sankyo. K.Tsujita received scholarship fund from Boehringer Ingelheim Japan. W.S. received grant from Nippon Boehringer Ingelheim, Daiichi Sankyo Company, Ltd., and received speaking honoraria from Nippon Boehringer Ingelheim, Daiichi Sankyo Company, Ltd.,Bristol-Meyers Squibb, Bayer Yakuhin, Pfizer, Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Novartis Pharma K.K., Johnson and Johnson KK, Medtronic Japan. H.T. received grant from Medtronic Japan Co., Ltd., Fukuda Denshi Kita-tohoku Hanbai Co., Ltd., BIOTRONIK Japan Co., Ltd., Japan Lifeline Co., and Boston Scientific Japan Co., Ltd., and received speaking honoraria from Boehringer Ingelheim. Masaya Watanabe received grant from Japan Society for the Promotion of Science and CASIO SCIENCE PROMOTION FOUNDATION. Masafumi Watanabe received grant from DAIICHI SANKYO COMPANY, LIMITED, CHUGAI PHARMACEUTICAL CO., LTD, Boston Scientific Corporation, Abbott Vascular Japan Co., Ltd, Cardinal Health, KANEKA MEDIX CORP, BIOTRONIK Japan, Inc, FUKUDA DENSHI, MEDTRONIC JAPAN CO., LTD, and received speaking honoraria from Otsuka Pharmaceutical Co., Ltd, DAIICHI SANKYO COMPANY, LIMITED, Nippon Boehringer Ingelheim Co., Ltd. T.Murohara received from Japan Boehringer Inc., Ono Pharmaceutical Inc., Daiichi- Sankyo Inc., AstraZeneca Inc. T.Kato received honoraria for lectures from Boehringer-Ingelheim, AstraZeneca, Ono Pharmaceutical, Medtronic, BIOTRONIK, Boston Scientific, Abbott. K.M. received grant from Daiichi-Sankyo, and received honoraria for lectures from Daiichi-Sankyo, Novartis, Takeda, Pfizer, Nippon Boehlinger ingelheim. K.N. received speaking honoraria from Astellas, Boehringer Ingelheim Japan, Daiichi Sankyo, Eli Lilly Japan, Kowa, Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Novartis Pharma, Ono Pharmaceutical, Takeda Pharmaceutical, MSD. T.Minamino received funding support for the present study, provision of study drugs and remuneration for lectures from Nippon Boehringer Ingelheim and Eli Lilly. K.I., K.Takahashi, M.O., S.O., M.S., T.Tokano, T.Kaneshiro, H.H., S.N., T.U., H.Y., K.O., T.Tanaka, N.K. declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

