Retinal peri-arteriolar versus peri-venular amyloidosis, hippocampal atrophy, and cognitive impairment: exploratory trial
- PMID: 38943220
- PMCID: PMC11212356
- DOI: 10.1186/s40478-024-01810-2
Retinal peri-arteriolar versus peri-venular amyloidosis, hippocampal atrophy, and cognitive impairment: exploratory trial
Abstract
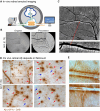
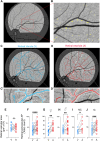
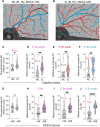
The relationship between amyloidosis and vasculature in cognitive impairment and Alzheimer's disease (AD) pathogenesis is increasingly acknowledged. We conducted a quantitative and topographic assessment of retinal perivascular amyloid plaque (AP) distribution in individuals with both normal and impaired cognition. Using a retrospective dataset of scanning laser ophthalmoscopy fluorescence images from twenty-eight subjects with varying cognitive states, we developed a novel image processing method to examine retinal peri-arteriolar and peri-venular curcumin-positive AP burden. We further correlated retinal perivascular amyloidosis with neuroimaging measures and neurocognitive scores. Our study unveiled that peri-arteriolar AP counts surpassed peri-venular counts throughout the entire cohort (P < 0.0001), irrespective of the primary, secondary, or tertiary vascular branch location, with a notable increase among cognitively impaired individuals. Moreover, secondary branch peri-venular AP count was elevated in the cognitively impaired (P < 0.01). Significantly, peri-venular AP count, particularly in secondary and tertiary venules, exhibited a strong correlation with clinical dementia rating, Montreal cognitive assessment score, hippocampal volume, and white matter hyperintensity count. In conclusion, our exploratory analysis detected greater peri-arteriolar versus peri-venular amyloidosis and a marked elevation of amyloid deposition in secondary branch peri-venular regions among cognitively impaired subjects. These findings underscore the potential feasibility of retinal perivascular amyloid imaging in predicting cognitive decline and AD progression. Larger longitudinal studies encompassing diverse populations and AD-biomarker confirmation are warranted to delineate the temporal-spatial dynamics of retinal perivascular amyloid deposition in cognitive impairment and the AD continuum.
Keywords: Amyloid imaging; Cognition; Hippocampal volume; Neurodegeneration; Peri-arteriolar; Peri-venular; Perivascular; Retina.
© 2024. The Author(s).
Conflict of interest statement
Koronyo-Hamaoui, Koronyo, Verdooner, and Black are co-founding members of NeuroVision Imaging Inc., 1395 Garden Highway, Suite 250, Sacramento, CA 95833, USA. Johnson and Verdooner are employed by NeuroVision Imaging Inc. NeuroVision’s sole involvement was to provide the processed retinal images. NeuroVision did not fund this study and was not involved in the development of the novel perivascular AP methodology or analysis. All authors declare that the research study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Update of
-
Distinctive retinal peri-arteriolar versus peri-venular amyloid plaque distribution correlates with the cognitive performance.bioRxiv [Preprint]. 2024 Feb 29:2024.02.27.580733. doi: 10.1101/2024.02.27.580733. bioRxiv. 2024. Update in: Acta Neuropathol Commun. 2024 Jun 28;12(1):109. doi: 10.1186/s40478-024-01810-2. PMID: 38464292 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

