The IDH paradox: Meta-analysis of alkylating chemotherapy in IDH-wild type and -mutant lower grade gliomas
- PMID: 38943513
- PMCID: PMC11449043
- DOI: 10.1093/neuonc/noae102
The IDH paradox: Meta-analysis of alkylating chemotherapy in IDH-wild type and -mutant lower grade gliomas
Abstract
Background: IDH-wild type (-wt) status is a prerequisite for the diagnosis of glioblastoma (GBM); however, IDH-wt gliomas with low-grade or anaplastic morphology have historically been excluded from GBM trials and may represent a distinct prognostic entity. While alkylating agent chemotherapy improves overall survival (OS) and progression-free survival (PFS) for IDH-wt GBM and also IDH-mutant gliomas, irrespective of grade, the benefit for IDH-wt diffuse histologic lower-grade gliomas is unclear.
Methods: We performed a meta-analysis of randomized clinical trials for World Health Organization (WHO) grades 2-3 gliomas (2009 to present) to determine the effect of alkylating chemotherapy on IDH-wt and -mutant gliomas using a random-effects model with inverse-variance pooling.
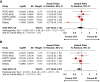
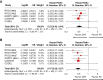
Results: We identified 6 trials with 1204 patients (430 IDH-wt, 774 IDH-mutant) that evaluated alkylating chemoradiotherapy versus radiotherapy alone, allowing us to perform an analysis focused on the value of adding alkylating chemotherapy to radiotherapy. For patients with IDH-wt tumors, alkylating chemotherapy added to radiotherapy was associated with improved PFS (HR:0.77 [95% CI: 0.62-0.97], P = .03) but not OS (HR:0.87 [95% CI: 0.64-1.18], P = .17). For patients with IDH-mutant tumors, alkylating chemotherapy added to radiotherapy improved both OS (HR:0.52 [95% CI: 0.42-0.64], P < .001) and PFS (HR = 0.47 [95% CI: 0.39-0.57], P < .001) compared to radiotherapy alone. The magnitude of benefit was similar for IDH-mutant gliomas with or without 1p19q-codeletion.
Conclusions: Alkylating chemotherapy reduces mortality by 48% and progression by 53% for patients with IDH-mutant gliomas. Optimal management of IDH-wt diffuse histologic lower-grade gliomas remains to be determined, as there is little evidence supporting an OS benefit from alkylating chemotherapy.
Keywords: alkylating chemotherapy; glioma; isocitrate-dehydrogenase; meta-analysis; radiotherapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Dr. Roy reports speaker honorarium from Varian and research grant from Swim Across America and the Prostate Cancer Foundation. Dr. Iwamoto has obtained grants or contracts through Columbia from Merck, Bristol Myers Squibb, Roche, Sapience, Novocure, Celldex, Tocagen, Forma, Celldex, and Northwest Biotherapeutics; is in consulting agreements with Novocure, Regeneron, Tocagen, Alexion Pharmaceuticals, Abbvie, Guidepoint Global, Merck, Kiyatec, PPD, Massive Bio, Medtronic, MimiVax, Gennao Bio, Ono, Anheart, Praesidia Therapeutics, and Xcures; has 2 US provisional patent applications (62/739,617 and 63/062,805) through Columbia University; received support for meetings and travel from Roche and Oncoceutics; and participates on advisory boards of Mimivax and Northwest Biotherapeutics. Dr. Brown receives honoraria from UpToDate. Dr. Bruce has a consulting agreement with Theracle. Dr. Kachnic reported receiving royalties from UpToDate and performing contracted research for Varian Medical Systems outside the submitted work. Dr. Neugut has consulted for Otsuka Pharmaceuticals, GlaxoSmithKline, Organon, Value Analytics, Merck, and United Biosource Corp. He has received grant support from Otsuka and Kyowa Kirin and was on the medical advisory board of EHE Intl. Dr. Yu receives speaking and consulting fees from RefleXion Medical, Boston Scientific, and Pfizer/Myovant and is an investor in Modifi Bio. Dr. Mehta reported receiving personal fees from Xoft, Novocure, Kazia, Telix, Zap, and stock ownership in Chimerix. Dr. Cheng reported receiving grants from Janssen and travel funding from Caris outside the submitted work. Dr. Wang reports personal fees and non-financial support from AbbVie, personal fees from Cancer Panels, personal fees from Doximity, personal fees and non-financial support from Elekta, personal fees and non-financial support from Merck, personal fees and non-financial support from Novocure, personal fees and non-financial support from RTOG Foundation, personal fees from Wolters Kluwer, grants and non-financial support from Genentech, grants and non-financial support from Varian, personal fees from Iylon Precision Oncology, outside the submitted work.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- van den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant Procarbazine, Lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2012;31(3):344–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

