Hyaluronic Acid Nanoparticles with Parameters Required for In Vivo Applications: From Synthesis to Parametrization
- PMID: 38943654
- PMCID: PMC11323013
- DOI: 10.1021/acs.biomac.4c00370
Hyaluronic Acid Nanoparticles with Parameters Required for In Vivo Applications: From Synthesis to Parametrization
Abstract
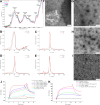
Hyaluronic acid is an excellent biocompatible material for in vivo applications. Its ability to bind CD44, a cell receptor involved in numerous biological processes, predetermines HA-based nanomaterials as unique carrier for therapeutic and theranostic applications. Although numerous methods for the synthesis of hyaluronic acid nanoparticles (HANPs) are available today, their low reproducibility and wide size distribution hinder the precise assessment of the effect on the organism. A robust and reproducible approach for producing HANPs that meet strict criteria for in vivo applications (e.g., to lung parenchyma) remains challenging. We designed and evaluated four protocols for the preparation of HANPs with those required parameters. The HA molecule was cross-linked by novel combinations of carbodiimide, and four different amine-containing compounds resulted in monodisperse HANPs with a low polydispersity index. By a complex postsynthetic characterization, we confirmed that the prepared HANPs meet the criteria for inhaled therapeutic delivery and other in vivo applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Haleem A.; Javaid M.; Singh R. P.; Rab S.; Suman R. Applications of Nanotechnology in Medical Field: A Brief Review. Global Health J. 2023, 7 (2), 70–77. 10.1016/j.glohj.2023.02.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

