A real-world disproportionality analysis of semaglutide: Post-marketing pharmacovigilance data
- PMID: 38943656
- PMCID: PMC11442840
- DOI: 10.1111/jdi.14229
A real-world disproportionality analysis of semaglutide: Post-marketing pharmacovigilance data
Abstract
Aim/introduction: The recent adverse reactions associated with semaglutide have led the Food and Drug Administration (FDA) to issue a "black box warning", and it is necessary to analyze all reports of adverse reactions to improve the safety of its clinical use.
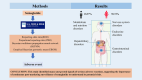
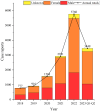
Materials and methods: Statistical analyses and signal mining were performed by obtaining the adverse event reports related to semaglutide in the FAERS database from the first quarter of 2018 to the fourth quarter of 2023. We used disproportionality and Bayesian analysis to examine clinical and demographic attributes, trends reported quarterly, and contrasts between two distinct indications (obesity and type 2 diabetes).
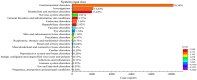
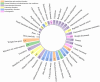
Results: We found 10 unexpected adverse signals related to "pancreatic cancer", "intestinal obstruction", "cholecystitis", and "polycystic ovary" and both the two different indications had the same serious adverse reaction events occurring.
Conclusions: This study identified many unexpected signals of serious adverse reactions, suggesting the importance of continuous post-marketing surveillance of semaglutide to understand its potential risks.
Keywords: Diabetes mellitus; Pharmacovigilance; Semaglutide.
© 2024 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors have no relevant financial or non‐financial interests to disclose.
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Figures
Similar articles
-
Risk of ophthalmic adverse drug reactions in patients prescribed glucagon-like peptide 1 receptor agonists: a pharmacovigilance study based on the FDA adverse event reporting system database.Endocrine. 2025 Apr;88(1):80-90. doi: 10.1007/s12020-024-04112-8. Epub 2024 Nov 22. Endocrine. 2025. PMID: 39578328
-
A post-marketing pharmacovigilance study of avapritinib: Adverse event data mining and analysis based on the United States Food and Drug Administration Adverse Event Reporting System database.Br J Clin Pharmacol. 2024 Aug;90(8):1816-1826. doi: 10.1111/bcp.15673. Epub 2023 Feb 6. Br J Clin Pharmacol. 2024. PMID: 36702463
-
The Cardiac and Renal Safety of Semaglutide in Patients with Type 2 Diabetes: A Real-World Study Based on FAERS.Cardiorenal Med. 2025;15(1):413-422. doi: 10.1159/000546238. Epub 2025 May 11. Cardiorenal Med. 2025. PMID: 40349689
-
Exploring Connections Between Weight-Loss Medications and Thyroid Cancer: A Look at the FDA Adverse Event Reporting System Database.Endocrinol Diabetes Metab. 2025 Mar;8(2):e70038. doi: 10.1002/edm2.70038. Endocrinol Diabetes Metab. 2025. PMID: 40055991 Free PMC article. Review.
-
Can Disproportionality Analysis of Post-marketing Case Reports be Used for Comparison of Drug Safety Profiles?Clin Drug Investig. 2017 May;37(5):415-422. doi: 10.1007/s40261-017-0503-6. Clin Drug Investig. 2017. PMID: 28224371 Review.
Cited by
-
Weight Reduction with GLP-1 Agonists and Paths for Discontinuation While Maintaining Weight Loss.Biomolecules. 2025 Mar 13;15(3):408. doi: 10.3390/biom15030408. Biomolecules. 2025. PMID: 40149944 Free PMC article. Review.
-
Glucagon-like peptide-1 receptor agonist-induced cholecystitis and cholelithiasis: a real-world pharmacovigilance analysis using the FAERS database.Front Pharmacol. 2025 Jul 8;16:1557691. doi: 10.3389/fphar.2025.1557691. eCollection 2025. Front Pharmacol. 2025. PMID: 40697657 Free PMC article.
-
A real-world disproportionality analysis of tirzepatide-related adverse events based on the FDA Adverse Event Reporting System (FAERS) database.Endocr J. 2025 Mar 3;72(3):273-283. doi: 10.1507/endocrj.EJ24-0286. Epub 2024 Nov 27. Endocr J. 2025. PMID: 39603650 Free PMC article.
-
Adverse drug reaction signals mining comparison of amiodarone and dronedarone: a pharmacovigilance study based on FAERS.Front Pharmacol. 2024 Oct 22;15:1438292. doi: 10.3389/fphar.2024.1438292. eCollection 2024. Front Pharmacol. 2024. PMID: 39502527 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2021B1515140012/Natural Science Foundation of Guangdong Province
- 2023A1515010083/Natural Science Foundation of Guangdong Province
- 20211800905342/Dongguan Science and Technology of Social Development Program
- k202005/Research and Development Fund of Dongguan People's Hospital
- 2021ZZDS006/Guangdong Medical University Students' Innovation Experiment Program
- 2021ZCDS003/Guangdong Medical University Students' Innovation Experiment Program
- 2022ZYDS003/Guangdong Medical University Students' Innovation Experiment Program
- 2022FYDB009/Guangdong Medical University Students' Innovation Experiment Program
- 2022FCDS003/Guangdong Medical University Students' Innovation Experiment Program
- GDMU2021003/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- GDMU2021049/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- GDMU2022031/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- GDMU2022047/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- GDMU2022063/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- GDMU2022077/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- GDMU2022078/Guangdong Medical University Students' Innovation and Entrepreneurship Training Program
- 202210571008/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- S202210571075/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- 202310571031/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- S202310571047/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- S202310571078/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- S202310571063/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- S202310571077/Provincial and National College Students' Innovation and Entrepreneurship Training Program
- 4SG24028G/Guangdong Medical University-Southern Medical University Twinning Research Team Project
- Cai Limin National Traditional Chinese Medicine Inheritance Studio
LinkOut - more resources
Full Text Sources
Medical

