Quercetin alleviates liver fibrosis via regulating glycolysis of liver sinusoidal endothelial cells and neutrophil infiltration
- PMID: 38943679
- PMCID: PMC11496877
- DOI: 10.17305/bb.2024.10530
Quercetin alleviates liver fibrosis via regulating glycolysis of liver sinusoidal endothelial cells and neutrophil infiltration
Abstract
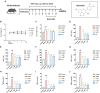
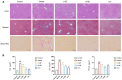
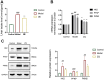
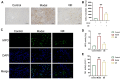
Liver fibrosis, a common characteristic in various chronic liver diseases, is largely influenced by glycolysis. Quercetin (QE), a natural flavonoid known to regulate glycolysis, was studied for its effects on liver fibrosis and its underlying mechanism. In a model of liver fibrosis induced by carbon tetrachloride (CCl4), we aimed to assess pathological features, serum marker levels, and analyze the expression of glycolysis-related enzymes at both mRNA and protein levels, with a focus on changes in liver sinusoidal endothelial cells (LSECs). Our results showed that QE effectively improved liver injury and fibrosis evident by improved pathological features and lowered levels of serum markers, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), total bile acid (TBA), total bilirubin (TBIL), direct bilirubin (DBIL), hyaluronic acid (HA), laminin (LN), and procollagen type III (PCIII). QE also decreased lactate production and downregulated the expression of glycolysis-related enzymes-pyruvate kinase M2 (PKM2), phosphofructokinase platelet (PFKP), and hexokinase II (HK2)-at both the mRNA and protein levels. QE reduced the expression and activity of these enzymes, resulting in reduced glucose consumption, adenosine triphosphate (ATP) production, and lactate generation. Further analysis revealed that QE inhibited the production of chemokine (C-X-C motif) ligand 1 (CXCL1) and suppressed neutrophil recruitment. Overall, QE showed promising therapeutic potential for liver fibrosis by targeting LSEC glycolysis and reducing neutrophil infiltration.
Conflict of interest statement
Conflicts of interest: Authors declare no conflicts of interest.
Figures

Similar articles
-
Integrative metabolomics and proteomics reveal the effect and mechanism of Zi Qi decoction on alleviating liver fibrosis.Sci Rep. 2024 Nov 22;14(1):28943. doi: 10.1038/s41598-024-80616-7. Sci Rep. 2024. PMID: 39578538 Free PMC article.
-
Mechanotransduction-induced glycolysis epigenetically regulates a CXCL1-dominant angiocrine signaling program in liver sinusoidal endothelial cells in vitro and in vivo.J Hepatol. 2022 Sep;77(3):723-734. doi: 10.1016/j.jhep.2022.03.029. Epub 2022 Apr 12. J Hepatol. 2022. PMID: 35421427 Free PMC article.
-
Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-кB/IкBα, p38 MAPK, and Bcl-2/Bax signaling.Int Immunopharmacol. 2017 Jun;47:126-133. doi: 10.1016/j.intimp.2017.03.029. Epub 2017 Apr 6. Int Immunopharmacol. 2017. PMID: 28391159
-
Comparison of four markers of hepatic fibrosis and hepatic function indices in patients with liver cirrhosis and hepatoma.Ann Palliat Med. 2021 Apr;10(4):4108-4121. doi: 10.21037/apm-20-1623. Epub 2021 Mar 24. Ann Palliat Med. 2021. PMID: 33832299
-
Hepatoprotective effect of total flavonoids of Mallotus apelta (Lour.) Muell.Arg. leaf against carbon tetrachloride-induced liver fibrosis in rats via modulation of TGF-β1/Smad and NF-κB signaling pathways.J Ethnopharmacol. 2020 May 23;254:112714. doi: 10.1016/j.jep.2020.112714. Epub 2020 Feb 24. J Ethnopharmacol. 2020. PMID: 32105750
Cited by
-
Lactate and lactylation in liver diseases: energy metabolism, inflammatory immunity and tumor microenvironment.Front Immunol. 2025 May 12;16:1581582. doi: 10.3389/fimmu.2025.1581582. eCollection 2025. Front Immunol. 2025. PMID: 40421024 Free PMC article. Review.
-
Crippled Hepatocarcinogenesis Inhibition of Quercetin in Glycolysis Pathway with Hepatic Farnesoid X Receptor Deficiency.Curr Pharm Des. 2025;31(22):1800-1815. doi: 10.2174/0113816128342642250111055339. Curr Pharm Des. 2025. PMID: 39917939
References
-
- Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. https://doi.org/10.1016/j.mam.2018.09.002. - PubMed
-
- Tan Z, Sun H, Xue T, Gan C, Liu H, Xie Y, et al. Liver fibrosis: therapeutic targets and advances in drug therapy. Front Cell Dev Biol. 2021;9:730176. https://doi.org/10.3389/fcell.2021.730176. - PMC - PubMed
-
- Cai X, Wang J, Wang J, Zhou Q, Yang B, He Q, et al. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: new insights into therapy. Pharmacol Res. 2020;155:104720. https://doi.org/10.1016/j.phrs.2020.104720. - PubMed
-
- Gong J, Gong H, Liu Y, Tao X, Zhang H. Calcipotriol attenuates liver fibrosis through the inhibition of vitamin D receptor-mediated NF-kappaB signaling pathway. Bioengineered. 2022;13(2):2658–72. https://doi.org/10.1080/21655979.2021.2024385. - PMC - PubMed
-
- Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–66. https://doi.org/10.1038/s41575-020-00372-7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

