Current Trends and Outcomes in Cellular Therapy Activity in the United States, Including Prospective Patient-Reported Outcomes Data Collection in the Center for International Blood and Marrow Transplant Research Registry
- PMID: 38944153
- PMCID: PMC11587342
- DOI: 10.1016/j.jtct.2024.06.021
Current Trends and Outcomes in Cellular Therapy Activity in the United States, Including Prospective Patient-Reported Outcomes Data Collection in the Center for International Blood and Marrow Transplant Research Registry
Abstract
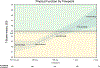
The Center for International Blood and Marrow Transplant Research (CIBMTR) prepares an annual set of summary slides to summarize the trends in transplantation and cellular therapies. For the first time in the 2023 summary slides, the CIBMTR incorporated data for patients receiving chimeric antigen receptor T cell (CAR-T) infusions. In addition, data on patient-reported outcomes (PROs) are included. This report aims to update the annual trends in US hematopoietic cell transplantation (HCT) activity and incorporate data on the use of CAR-T therapies. A second aim is to present and describe the development, implementation, and current status of PRO data collection. In August 2020, the CIBMTR launched the Protocol for Collection of Patient-Reported Outcomes Data (CIBMTR PRO Protocol). The CIBMTR PRO Protocol operates under a centralized infrastructure to reduce the burden to centers. Specifically, PRO data are collected from a prospective convenience sample of adult HCT and CAR-T recipients who received treatment at contributing centers and consented for research. Data are merged and stored with the clinical data and used under the governance of the CIBMTR Research Database Protocol. Participants answer a series of surveys developed by the Patient Reported Outcomes Measurement Information System (PROMIS) focusing on physical, social and emotional, and other measures assessing financial well-being, occupational functioning, and social determinants of health. To complement traditionally measured clinical outcomes, the surveys are administered at the same time points at which clinical data are routinely collected. As of September 2023, PRO data have been collected from 993 patients across 25 different centers. With the goal of incorporating these important patient perspectives into standard clinical care, the CIBMTR has added the PRO data to Data Back to Centers (DBtC). Through expanding the data types represented in the registry, the CIBMTR aims to support holistic research accounting for the patients' perspective in improving patient outcomes. CIBMTR PRO data aim to provide a foundation for future large-scale, population-level evaluations to identify areas for improvement, emerging disparities in access and health outcomes (eg, by age, race, and ethnicity), and new therapies that may impact current treatment guidelines. Continuing to collect and grow the PRO data is critical for understanding these changes and identifying methods for improving patients' quality of life.
Keywords: Patient-reported outcomes; Quality of life; Self-reported well being.
Copyright © 2024 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Rachel Phelan reports compensation from bluebird bio: advisory board; Amgen: research funding (ended December 2021).
Dr. Jeffery Auletta reports Government COI.
Dr. Steve Devine reports compensation – consulting payments from Bristol Myers Squibb and Sanofi and consulting with Bristol Myers Squibb and Sanofi.
Dr. Mehdi Hamadani reports Research Funding/Support from ADC Therapeutics and Astellas Pharma; Consultancy from ADC Therapeutics, Abbvie, Genmab, CRISPR, Allovir, Cariou, Autolus, and Forte Biosciences; Speaker’s Bureau from ADC
Therapeutics, Kite, AstraZeneca, and BeiGene; and DMC from Myeloid Therapeutics and CRISPR.
Dr. Stephanie J. Lee reports Compensation: SJL has received consulting fees from Mallinckrodt, Equillium, Kadmon, Novartis, Incyte; research funding from AstraZeneca, Incyte, Kadmon, Pfizer, Sanofi and Syndax, and drug supply from Janssen. She is on clinical trial steering committees for Incyte and Sanofi. She is on the Board of Directors of the National Marrow Donor Program (uncompensated)
Significant Payments: Sanofi consultant between $5,000-$10,000 in 2023
Relationships: NMDP Board of Directors (uncompensated)
Proprietary: Licenses for the Lee Symptom Scale, paid to her institution, not to her personally.
Dr. Marcelo Pasquini reports Consultancy and Research Fudnign for Bristol Myers Squibb and Research Funding from Novartis, Kite and Janssen.
Dr. Doug Rizzo reports Compensation: I participate annually as an external consultant for Optum Stem Cell Transplant Expert Panel to provide input on their center of excellence criteria, updating indications for HCT and CT, and other advice as requested. Payment of $1000 is directed to MCW on my behalf. No MCW or CIBMTR proprietary information is shared with Optum.
Relationships: Expert panel for HCT for Optum as outlined in item 1 (Compensation)
Other Interests: As a faculty member of MCW and clinician in the F&MCW HCT/ CT program I have a potential COI with my role as a senior scientific director of the CIBMTR. To avoid conflicts of interest I specifically do not provide direction to the CIBMTR Audit program as pertains to F&MCW program or other local potentially competitive HCT programs that affects audit results. Audits of F&MCW HCT program also include a third party observer. As well, to avoid COI regarding the center specific survival analysis, all decisions involving data preparation and analysis that I make are blinded with regard to individual center identities until the analyses are completed and ready for submission to HRSA.
Dr. Bronwen Shaw reports Relations as a consultant with Orcabio and Mallinkrodt.
Dr. Kathryn Flynn reports Consulting Fees from Inhibikase, Novartis, and Pfizer as well as Research Funding from NIH and Novartis.
Dr. Samantha Jaglowski reports Advisory Board Funding from CRISPR Therapeutics.
Figures



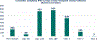
References
-
- Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims | FDA [Internet]. [cited 2022 Jan 10];Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- Fayers PM, Machin D. Quality of Life: The Assessment, Analysis and Interpretation of Patient-reported Outcomes [Internet]. John Wiley & Sons; 2013. Available from: https://play.google.com/store/books/details?id=pqX6WKgHKJsC
-
- Xiao C, Polomano R, Bruner DW. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs [Internet] 2013. [cited 2024 Mar 27];36(6). Available from: https://journals.lww.com/cancernursingonline/fulltext/2013/11000/compari... - PubMed
-
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol [Internet] 2010;63(11):1179–94. Available from: 10.1016/j.jclinepi.2010.04.011 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

