Potential Psychosis Induced by a Sustained High Plasma Levodopa Concentration Due to Continuous Subcutaneous Foslevodopa/Foscarbidopa Infusion in a Patient With Parkinson's Disease: A Case Report
- PMID: 38945151
- PMCID: PMC11540536
- DOI: 10.14802/jmd.24114
Potential Psychosis Induced by a Sustained High Plasma Levodopa Concentration Due to Continuous Subcutaneous Foslevodopa/Foscarbidopa Infusion in a Patient With Parkinson's Disease: A Case Report
Conflict of interest statement
The authors have no financial conflicts of interest.
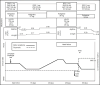
Figures
References
-
- Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–560. - PubMed
-
- Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1–8. - PubMed
-
- Soileau MJ, Aldred J, Budur K, Fisseha N, Fung VS, Jeong A, et al. Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol. 2022;21:1099–1109. - PubMed
LinkOut - more resources
Full Text Sources

