Systemic Deletion of ARRDC4 Improves Cardiac Reserve and Exercise Capacity in Diabetes
- PMID: 38946541
- PMCID: PMC11257811
- DOI: 10.1161/CIRCRESAHA.123.323158
Systemic Deletion of ARRDC4 Improves Cardiac Reserve and Exercise Capacity in Diabetes
Abstract
Background: Exercise intolerance is an independent predictor of poor prognosis in diabetes. The underlying mechanism of the association between hyperglycemia and exercise intolerance remains undefined. We recently demonstrated that the interaction between ARRDC4 (arrestin domain-containing protein 4) and GLUT1 (glucose transporter 1) regulates cardiac metabolism.
Methods: To determine whether this mechanism broadly impacts diabetic complications, we investigated the role of ARRDC4 in the pathogenesis of diabetic cardiac/skeletal myopathy using cellular and animal models.
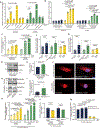
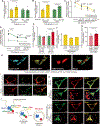
Results: High glucose promoted translocation of MondoA into the nucleus, which upregulated Arrdc4 transcriptional expression, increased lysosomal GLUT1 trafficking, and blocked glucose transport in cardiomyocytes, forming a feedback mechanism. This role of ARRDC4 was confirmed in human muscular cells from type 2 diabetic patients. Prolonged hyperglycemia upregulated myocardial Arrdc4 expression in multiple types of mouse models of diabetes. We analyzed hyperglycemia-induced cardiac and skeletal muscle abnormalities in insulin-deficient mice. Hyperglycemia increased advanced glycation end-products and elicited oxidative and endoplasmic reticulum stress leading to apoptosis in the heart and peripheral muscle. Deletion of Arrdc4 augmented tissue glucose transport and mitochondrial respiration, protecting the heart and muscle from tissue damage. Stress hemodynamic analysis and treadmill exhaustion test uncovered that Arrdc4-knockout mice had greater cardiac inotropic/chronotropic reserve with higher exercise endurance than wild-type animals under diabetes. While multiple organs were involved in the mechanism, cardiac-specific overexpression using an adenoassociated virus suggests that high levels of myocardial ARRDC4 have the potential to contribute to exercise intolerance by interfering with cardiac metabolism through its interaction with GLUT1 in diabetes. Importantly, the ARRDC4 mutation mouse line exhibited greater exercise tolerance, showing the potential therapeutic impact on diabetic cardiomyopathy by disrupting the interaction between ARRDC4 and GLUT1.
Conclusions: ARRDC4 regulates hyperglycemia-induced toxicities toward cardiac and skeletal muscle, revealing a new molecular framework that connects hyperglycemia to cardiac/skeletal myopathy to exercise intolerance.
Keywords: adaptor protein; diabetic cardiomyopathy; energy metabolism; exercise endurance; health.
Conflict of interest statement
None.
Figures





Similar articles
-
Interaction of ARRDC4 With GLUT1 Mediates Metabolic Stress in the Ischemic Heart.Circ Res. 2022 Sep 2;131(6):510-527. doi: 10.1161/CIRCRESAHA.122.321351. Epub 2022 Aug 11. Circ Res. 2022. PMID: 35950500 Free PMC article.
-
Cardiomyocyte-specific Txnip C247S mutation improves left ventricular functional reserve in streptozotocin-induced diabetic mice.Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H259-H274. doi: 10.1152/ajpheart.00174.2021. Epub 2021 Jun 4. Am J Physiol Heart Circ Physiol. 2021. PMID: 34085839 Free PMC article.
-
Glucose transporter 1 is essential to maintain brain endothelial cell homeostasis under hyperglycemia condition.Am J Physiol Cell Physiol. 2025 Aug 1;329(2):C341-C354. doi: 10.1152/ajpcell.00615.2024. Epub 2025 Jun 23. Am J Physiol Cell Physiol. 2025. PMID: 40549566 Free PMC article.
-
Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review.Hum Reprod Update. 2018 Jan 1;24(1):86-105. doi: 10.1093/humupd/dmx033. Hum Reprod Update. 2018. PMID: 29136166
-
Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes: a systematic review and economic modelling.Health Technol Assess. 2024 Dec;28(80):1-190. doi: 10.3310/JYPL3536. Health Technol Assess. 2024. PMID: 39673446 Free PMC article.
References
-
- Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH and Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. - PubMed
-
- Bertoni AG, Tsai A, Kasper EK and Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

