Unraveling the Pathogenesis of Post-Stroke Depression in a Hemorrhagic Mouse Model through Frontal Lobe Circuitry and JAK-STAT Signaling
- PMID: 38946585
- PMCID: PMC11434213
- DOI: 10.1002/advs.202402152
Unraveling the Pathogenesis of Post-Stroke Depression in a Hemorrhagic Mouse Model through Frontal Lobe Circuitry and JAK-STAT Signaling
Abstract
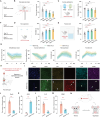
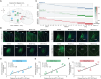
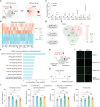
Post-stroke depression is a common complication that imposes significant burdens and challenges on patients. The occurrence of depression is often associated with frontal lobe hemorrhage, however, current understanding of the underlying mechanisms remains limited. Here, the pathogenic mechanisms associated with the circuitry connectivity, electrophysiological alterations, and molecular characteristics are investigated related to the frontal lobe in adult male mice following unilateral injection of blood in the medial prefrontal cortex (mPFC). It is demonstrated that depression is a specific neurological complication in the unilateral hematoma model of the mPFC, and the ventral tegmental area (VTA) shows a higher percentage of connectivity disruption compared to the lateral habenula (LHb) and striatum (STR). Additionally, long-range projections originating from the frontal lobe demonstrate higher damage percentages within the connections between each region and the mPFC. mPFC neurons reveal reduced neuronal excitability and altered synaptic communication. Furthermore, transcriptomic analysis identifies the involvement of the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway, and targeting the JAK-STAT pathway significantly alleviates the severity of depressive symptoms. These findings improve the understanding of post-hemorrhagic depression and may guide the development of efficient treatments.
Keywords: circuitry connectivity; electrophysiological alterations; frontal lobe hemorrhage; hematoma volumes; post‐stroke depression.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Canonical JAK-STAT signaling is pivotal for long-term depression at adult hippocampal temporoammonic-CA1 synapses.FASEB J. 2017 Aug;31(8):3449-3466. doi: 10.1096/fj.201601293RR. Epub 2017 May 1. FASEB J. 2017. PMID: 28461339
-
Three-needle electroacupuncture ameliorates depressive-like behaviors in a mouse model of post-stroke depression by promoting excitatory synapse formation via the NGL-3/L1cam pathway.Brain Res. 2024 Oct 15;1841:149087. doi: 10.1016/j.brainres.2024.149087. Epub 2024 Jun 11. Brain Res. 2024. PMID: 38871241
-
JAK/STAT pathway regulation of GABAA receptor expression after differing severities of experimental TBI.Exp Neurol. 2015 Sep;271:445-56. doi: 10.1016/j.expneurol.2015.07.001. Epub 2015 Jul 11. Exp Neurol. 2015. PMID: 26172316 Free PMC article.
-
The JAK/STAT signaling pathway: from bench to clinic.Signal Transduct Target Ther. 2021 Nov 26;6(1):402. doi: 10.1038/s41392-021-00791-1. Signal Transduct Target Ther. 2021. PMID: 34824210 Free PMC article. Review.
-
Targeting the JAK-STAT pathway in autoimmune diseases and cancers: A focus on molecular mechanisms and therapeutic potential.Biochem Pharmacol. 2021 Nov;193:114760. doi: 10.1016/j.bcp.2021.114760. Epub 2021 Sep 4. Biochem Pharmacol. 2021. PMID: 34492272 Review.
Cited by
-
fNIRS evidence of abnormal frontotemporal cortex activation and functional connectivity in depressed patients after stroke: neuromodulatory mechanisms from mild to moderate depression.Front Neurol. 2025 Jul 18;16:1599733. doi: 10.3389/fneur.2025.1599733. eCollection 2025. Front Neurol. 2025. PMID: 40757031 Free PMC article.
-
S-ketamine Alleviates Neuroinflammation and Attenuates Lipopolysaccharide-Induced Depression Via Targeting SIRT2.Adv Sci (Weinh). 2025 Jun;12(23):e2416481. doi: 10.1002/advs.202416481. Epub 2025 Apr 2. Adv Sci (Weinh). 2025. PMID: 40171943 Free PMC article.
-
Prevalence and Associated Factors of Depression in Patients with Ischaemic Stroke: A Cross-Sectional Study.Neuropsychiatr Dis Treat. 2025 Apr 14;21:875-883. doi: 10.2147/NDT.S514184. eCollection 2025. Neuropsychiatr Dis Treat. 2025. PMID: 40256205 Free PMC article.
References
-
- Burvill P. W., Johnson G. A., Jamrozik K. D., Anderson C. S., Stewart‐Wynne E. G., Chakera T. M., Br J. Psychiatry 1995, 166, 328. - PubMed
-
- Balami J. S., Buchan A. M., Lancet Neurol. 2012, 11, 101. - PubMed
-
- a) Keins S., Abramson J. R., Mallick A., Castello J. P., Rodriguez‐Torres A., Popescu D., Hoffman D., Kourkoulis C., Gurol M. E., Greenberg S. M., Anderson C. D., Viswanathan A., Rosand J., Biffi A., Stroke 2023, 54, 105; - PMC - PubMed
- b) Kubiszewski P., Sugita L., Kourkoulis C., DiPucchio Z., Schwab K., Anderson C. D., Gurol M. E., Greenberg S. M., Viswanathan A., Rosand J., Biffi A., JAMA Neurol. 2020, 78, 61; - PMC - PubMed
- c) Stern‐Nezer S., Eyngorn I., Mlynash M., Snider R. W., Venkatsubramanian C., Wijman C. A. C., Buckwalter M. S., NeuroRehabilitation 2017, 41, 179. - PubMed
-
- Oestreich L. K., Lo J. W., Di Biase M. A., Sachdev P. S., Mok A. H., Wright P., Crawford J. D., Lam B., Traykov L., Kohler S., Staals J. E., van Oostenbrugge R., Chen C., Desmond D. W., Yu K. H., Lee M., Klimkowicz‐Mrowiec A., Bordet R., O'Sullivan M. J., Zalesky A., Psychiatry Clin. Neurosci. 2023, 78, 229. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
