Unraveling the Pathogenesis of Post-Stroke Depression in a Hemorrhagic Mouse Model through Frontal Lobe Circuitry and JAK-STAT Signaling
- PMID: 38946585
- PMCID: PMC11434213
- DOI: 10.1002/advs.202402152
Unraveling the Pathogenesis of Post-Stroke Depression in a Hemorrhagic Mouse Model through Frontal Lobe Circuitry and JAK-STAT Signaling
Abstract
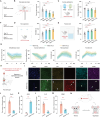
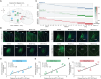
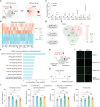
Post-stroke depression is a common complication that imposes significant burdens and challenges on patients. The occurrence of depression is often associated with frontal lobe hemorrhage, however, current understanding of the underlying mechanisms remains limited. Here, the pathogenic mechanisms associated with the circuitry connectivity, electrophysiological alterations, and molecular characteristics are investigated related to the frontal lobe in adult male mice following unilateral injection of blood in the medial prefrontal cortex (mPFC). It is demonstrated that depression is a specific neurological complication in the unilateral hematoma model of the mPFC, and the ventral tegmental area (VTA) shows a higher percentage of connectivity disruption compared to the lateral habenula (LHb) and striatum (STR). Additionally, long-range projections originating from the frontal lobe demonstrate higher damage percentages within the connections between each region and the mPFC. mPFC neurons reveal reduced neuronal excitability and altered synaptic communication. Furthermore, transcriptomic analysis identifies the involvement of the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway, and targeting the JAK-STAT pathway significantly alleviates the severity of depressive symptoms. These findings improve the understanding of post-hemorrhagic depression and may guide the development of efficient treatments.
Keywords: circuitry connectivity; electrophysiological alterations; frontal lobe hemorrhage; hematoma volumes; post‐stroke depression.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Burvill P. W., Johnson G. A., Jamrozik K. D., Anderson C. S., Stewart‐Wynne E. G., Chakera T. M., Br J. Psychiatry 1995, 166, 328. - PubMed
-
- Balami J. S., Buchan A. M., Lancet Neurol. 2012, 11, 101. - PubMed
-
- a) Keins S., Abramson J. R., Mallick A., Castello J. P., Rodriguez‐Torres A., Popescu D., Hoffman D., Kourkoulis C., Gurol M. E., Greenberg S. M., Anderson C. D., Viswanathan A., Rosand J., Biffi A., Stroke 2023, 54, 105; - PMC - PubMed
- b) Kubiszewski P., Sugita L., Kourkoulis C., DiPucchio Z., Schwab K., Anderson C. D., Gurol M. E., Greenberg S. M., Viswanathan A., Rosand J., Biffi A., JAMA Neurol. 2020, 78, 61; - PMC - PubMed
- c) Stern‐Nezer S., Eyngorn I., Mlynash M., Snider R. W., Venkatsubramanian C., Wijman C. A. C., Buckwalter M. S., NeuroRehabilitation 2017, 41, 179. - PubMed
-
- Oestreich L. K., Lo J. W., Di Biase M. A., Sachdev P. S., Mok A. H., Wright P., Crawford J. D., Lam B., Traykov L., Kohler S., Staals J. E., van Oostenbrugge R., Chen C., Desmond D. W., Yu K. H., Lee M., Klimkowicz‐Mrowiec A., Bordet R., O'Sullivan M. J., Zalesky A., Psychiatry Clin. Neurosci. 2023, 78, 229. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
