CircORC2 promoted proliferation and inhibited the sensitivity of osteosarcoma cell lines to cisplatin by regulating the miR-485-3p/TRIM2 axis
- PMID: 38946721
- PMCID: PMC11208123
- DOI: 10.1002/ccs3.12029
CircORC2 promoted proliferation and inhibited the sensitivity of osteosarcoma cell lines to cisplatin by regulating the miR-485-3p/TRIM2 axis
Abstract
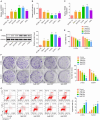
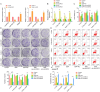
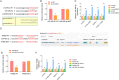
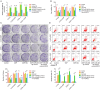
Resistance to chemotherapy leads to poor prognosis for osteosarcoma (OS) patients. However, due to the high metastasis of tumor and the decrease in sensitivity of tumor cells to cisplatin (DDP), the 5-year survival rate of OS patients is still unsatisfactory. This study explored a mechanism for improving the sensitivity of OS cells to DDP. A DDP-resistant OS cell model was established, and we have found that circORC2 and TRIM2 were upregulated in DDP-resistant OS cells, but miR-485-3p was downregulated. The cell viability and proliferation of the OS cells decreased gradually with the increase of DDP dose, but a gradual increase in apoptosis was noted. CircORC2 promoted OS cell proliferation and DDP resistance and upregulated TRIM2 expression by targeting miR-485-3p. Functionally, circORC2 downregulated miR-485-3p to promote OS cell proliferation and inhibit DDP sensitivity. Additionally, it promoted cell proliferation and inhibited the sensitivity of DDP by regulating the miR-485-3p/TRIM2 axis. In conclusion, circORC2 promoted cell proliferation and inhibited the DDP sensitivity in OS cells via the miR-485-3p/TRIM2 axis. These findings indicated the role of circORC2 in regulating the sensitivity of OS cells to DDP.
Keywords: MiR‐485‐3p; TRIM2; circORC2; cisplatin resistance; osteosarcoma.
© 2024 The Authors. Journal of Cell Communication and Signaling published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Similar articles
-
Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p.Eur Rev Med Pharmacol Sci. 2019 Sep;23(17):7256-7265. doi: 10.26355/eurrev_201909_18828. Eur Rev Med Pharmacol Sci. 2019. PMID: 31539112
-
HOTAIR Promotes Cisplatin Resistance of Osteosarcoma Cells by Regulating Cell Proliferation, Invasion, and Apoptosis via miR-106a-5p/STAT3 Axis.Cell Transplant. 2020 Jan-Dec;29:963689720948447. doi: 10.1177/0963689720948447. Cell Transplant. 2020. PMID: 32757663 Free PMC article.
-
CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis.Mol Cancer. 2021 Dec 7;20(1):161. doi: 10.1186/s12943-021-01453-0. Mol Cancer. 2021. PMID: 34876132 Free PMC article.
-
LncRNA NORAD targets miR-410-3p to regulate drug resistance sensitivity of osteosarcoma.Cell Mol Biol (Noisy-le-grand). 2020 Jun 5;66(3):143-148. Cell Mol Biol (Noisy-le-grand). 2020. PMID: 32538761
-
Long non-coding RNA long intergenic non-coding 00641 mediates cell progression with stimulating cisplatin-resistance in osteosarcoma cells via microRNA-320d/myeloid cell leukemia-1 axis.Bioengineered. 2022 Mar;13(3):7238-7252. doi: 10.1080/21655979.2022.2045090. Bioengineered. 2022. PMID: 35266447 Free PMC article.
References
-
- Biazzo, A. , and De Paolis M.. 2016. “Multidisciplinary Approach to Osteosarcoma.” Acta Orthopaedica Belgica 82(4): 690–698. - PubMed
-
- Li, Zhihong , Dou Pengcheng, Liu Tang, and He Shasha. 2017. “Application of Long Noncoding RNAs in Osteosarcoma: Biomarkers and Therapeutic Targets.” Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 42(4): 1407–1419. 10.1159/000479205. - DOI - PubMed
-
- Liu, Fengsong , Wang Kai, Zhang Liang, and Yang Ya‑Lin. 2018. “Bone Morphogenetic Protein and Activin Membrane‐Bound Inhibitor Suppress Bone Cancer Progression in MG63 and SAOS Cells via Regulation of the TGF‐β‐Induced EMT Signaling Pathway.” Oncology Letters 16(4): 5113–5121. 10.3892/ol.2018.9268. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

