Ferrocenyl-triazole complexes and their use in heavy metal cation sensing
- PMID: 38946768
- PMCID: PMC11211737
- DOI: 10.1039/d4ra04023f
Ferrocenyl-triazole complexes and their use in heavy metal cation sensing
Abstract
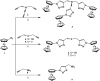
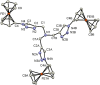
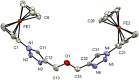
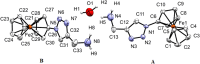
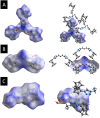
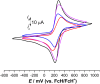
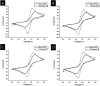
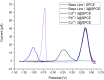
Complexes tris((1-ferrocenyl-1H-1,2,3-triazol-4-yl)methyl)amine (3), bis((1-ferrocenyl-1H-1,2,3-triazol-4-yl)methyl)amine (6), bis((1-ferrocenyl-1H-1,2,3-triazol-4-yl)methyl)ether (7), and 1-ferrocenyl-1H-1,2,3-triazol-4-yl)methanamine (9) were synthesized using the copper-catalyzed click reaction. Complexes 3, 6, 7, and 9 were characterized using NMR (1H and 13{1H}) and IR spectroscopy, elemental analysis, and mass spectrometry. Structures of 3, 7, and 9 in the solid state were determined using single-crystal X-ray diffraction. It was found that the triazole rings were planar and slightly twisted with respect to the cyclopentadienyl groups attached to them. Chains and 3D network structures were observed due to the presence of π⋯π and C-H⋯N interactions between the cyclopentadienyl and triazole ligands. A reversible redox behavior of the Fc groups between 239 and 257 mV with multicycle stability was characteristic for all the compounds, revealing that the electrochemically generated species Fc+ remained soluble in dichloromethane. Electrochemical sensor tests demonstrated the applicability of all the complexes to enhance the quantification sensing behavior of the screen-printed carbon electrode (SPCE) toward Cd2+, Pb2+, and Cu2+ ions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis, crystal structures and luminescence properties of seven mononuclear zinc(II), cadmium(II), cobalt(II) and nickel(II) complexes with 5-(4-methylphenyl)-3-(pyridin-2-yl)-1H-1,2,4-triazole.Acta Crystallogr C Struct Chem. 2017 May 1;73(Pt 5):382-392. doi: 10.1107/S2053229617004697. Epub 2017 Apr 11. Acta Crystallogr C Struct Chem. 2017. PMID: 28469064
-
Photoluminescent copper(I) complexes with amido-triazolato ligands.Inorg Chem. 2011 Apr 18;50(8):3431-41. doi: 10.1021/ic102338g. Epub 2011 Mar 21. Inorg Chem. 2011. PMID: 21417454
-
Facile synthesis and bonding of 4-ferrocenyl-1,2,4-triazol-5-ylidene complexes.Dalton Trans. 2024 Jul 9;53(27):11445-11453. doi: 10.1039/d4dt01433b. Dalton Trans. 2024. PMID: 38904982
-
A series of new polyoxometalate-based metal-organic complexes with different rigid pyridyl-bis(triazole) ligands: assembly, structures and electrochemical properties.RSC Adv. 2018 Jun 20;8(40):22676-22686. doi: 10.1039/c8ra03277g. eCollection 2018 Jun 19. RSC Adv. 2018. PMID: 35539734 Free PMC article.
-
Crystal structures of 2,6-bis-[(1H-1,2,4-triazol-1-yl)meth-yl]pyridine and 1,1-[pyridine-2,6-diylbis(methyl-ene)]bis-(4-methyl-1H-1,2,4-triazol-4-ium) iodide triiodide.Acta Crystallogr E Crystallogr Commun. 2015 Jan 3;71(Pt 2):128-32. doi: 10.1107/S2056989014027881. eCollection 2015 Feb 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25878799 Free PMC article.
References
-
- Stahl A. Lazar A. I. Muchemu V. N. Nau W. M. Ullrich M. S. Hennig A. Anal. Bioanal. Chem. 2017;409:6485–6494. - PubMed
-
- Khudyakova S. N. Vishnikin A. B. Smityuk N. M. Int. J. Environ. Anal. Chem. 2018;98:1253–1273.
-
- Karawek A. Srisuwannaket C. Mayurachayakul P. Sukwattanasinitt M. Pratumyot K. Dilokpramuan A. Mingvanish W. Kidkhunthod P. Niamnont N. Color. Technol. 2022;138:38–46.
-
- Wang X. Qi Y. Shen Y. Yuan Y. Zhang L. Zhang C. Sun Y. Sens. Actuators, B. 2020;310:127756.
-
- Wan J. Shen Y. Xu L. Xu R. Zhang J. Sun H. Zhang C. Yin C. Wang X. J. Electroanal. Chem. 2021;895:115374.
LinkOut - more resources
Full Text Sources
Miscellaneous

