Role of gut-liver axis and glucagon-like peptide-1 receptor agonists in the treatment of metabolic dysfunction-associated fatty liver disease
- PMID: 38946874
- PMCID: PMC11212696
- DOI: 10.3748/wjg.v30.i23.2964
Role of gut-liver axis and glucagon-like peptide-1 receptor agonists in the treatment of metabolic dysfunction-associated fatty liver disease
Abstract
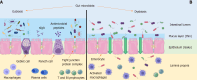
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a hepatic manifestation of the metabolic syndrome. It is one of the most common liver diseases worldwide and shows increasing prevalence rates in most countries. MAFLD is a progressive disease with the most severe cases presenting as advanced fibrosis or cirrhosis with an increased risk of hepatocellular carcinoma. Gut microbiota play a significant role in the pathogenesis and progression of MAFLD by disrupting the gut-liver axis. The mechanisms involved in maintaining gut-liver axis homeostasis are complex. One critical aspect involves preserving an appropriate intestinal barrier permeability and levels of intestinal lumen metabolites to ensure gut-liver axis functionality. An increase in intestinal barrier permeability induces metabolic endotoxemia that leads to steatohepatitis. Moreover, alterations in the absorption of various metabolites can affect liver metabolism and induce liver steatosis and fibrosis. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a class of drugs developed for the treatment of type 2 diabetes mellitus. They are also commonly used to combat obesity and have been proven to be effective in reversing hepatic steatosis. The mechanisms reported to be involved in this effect include an improved regulation of glycemia, reduced lipid synthesis, β-oxidation of free fatty acids, and induction of autophagy in hepatic cells. Recently, multiple peptide receptor agonists have been introduced and are expected to increase the effectiveness of the treatment. A modulation of gut microbiota has also been observed with the use of these drugs that may contribute to the amelioration of MAFLD. This review presents the current understanding of the role of the gut-liver axis in the development of MAFLD and use of members of the GLP-1 RA family as pleiotropic agents in the treatment of MAFLD.
Keywords: Bariatric surgery; Gastrointestinal microbiota; Glucagon-like peptide-1; Glucagon-like peptide-2; Metabolic dysfunction-associated fatty liver disease; Metabolic dysfunction-associated steatohepatitis; Metabolic syndrome; Non-alcoholic fatty liver disease; Non-alcoholic steatohepatitis; Obesity.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest related to this article.
Figures

References
-
- Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672–2682. - PubMed
-
- Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

