In vivo modeling recapitulates radiotherapy delivery and late-effect profile for childhood medulloblastoma
- PMID: 38946880
- PMCID: PMC11212071
- DOI: 10.1093/noajnl/vdae091
In vivo modeling recapitulates radiotherapy delivery and late-effect profile for childhood medulloblastoma
Abstract
Background: Medulloblastoma (MB) is the most common malignant pediatric brain tumor, with 5-year survival rates > 70%. Cranial radiotherapy (CRT) to the whole brain, with posterior fossa boost (PFB), underpins treatment for non-infants; however, radiotherapeutic insult to the normal brain has deleterious consequences to neurocognitive and physical functioning, and causes accelerated aging/frailty. Approaches to ameliorate radiotherapy-induced late-effects are lacking and a paucity of appropriate model systems hinders their development.
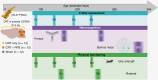
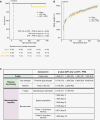
Methods: We have developed a clinically relevant in vivo model system that recapitulates the radiotherapy dose, targeting, and developmental stage of childhood medulloblastoma. Consistent with human regimens, age-equivalent (postnatal days 35-37) male C57Bl/6J mice received computerized tomography image-guided CRT (human-equivalent 37.5 Gy EQD2, n = 12) ± PFB (human-equivalent 48.7 Gy EQD2, n = 12), via the small animal radiation research platform and were longitudinally assessed for > 12 months.
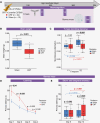
Results: CRT was well tolerated, independent of PFB receipt. Compared to a sham-irradiated group (n = 12), irradiated mice were significantly frailer following irradiation (frailty index; P = .0002) and had reduced physical functioning; time to fall from a rotating rod (rotarod; P = .026) and grip strength (P = .006) were significantly lower. Neurocognitive deficits were consistent with childhood MB survivors; irradiated mice displayed significantly worse working memory (Y-maze; P = .009) and exhibited spatial memory deficits (Barnes maze; P = .029). Receipt of PFB did not induce a more severe late-effect profile.
Conclusions: Our in vivo model mirrored childhood MB radiotherapy and recapitulated features observed in the late-effect profile of MB survivors. Our clinically relevant model will facilitate both the elucidation of novel/target mechanisms underpinning MB late effects and the development of novel interventions for their amelioration.
Keywords: late-effects; medulloblastoma; modelling; radiotherapy; survivorship.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques?Int J Radiat Oncol Biol Phys. 2005 Oct 1;63(2):362-72. doi: 10.1016/j.ijrobp.2005.01.060. Int J Radiat Oncol Biol Phys. 2005. PMID: 16168831
-
Comparison of neuropsychological functioning in pediatric posterior fossa tumor survivors: Medulloblastoma, low-grade astrocytoma, and healthy controls.Pediatr Blood Cancer. 2022 Feb;69(2):e29491. doi: 10.1002/pbc.29491. Epub 2021 Nov 29. Pediatr Blood Cancer. 2022. PMID: 34842359 Free PMC article.
-
Protracted radiotherapy treatment duration in medulloblastoma.Am J Clin Oncol. 2003 Feb;26(1):55-9. doi: 10.1097/00000421-200302000-00012. Am J Clin Oncol. 2003. PMID: 12576926
-
High dose craniospinal irradiation as independent risk factor of permanent alopecia in childhood medulloblastoma survivors: cohort study and literature review.J Neurooncol. 2022 Dec;160(3):659-668. doi: 10.1007/s11060-022-04186-2. Epub 2022 Nov 12. J Neurooncol. 2022. PMID: 36369416 Free PMC article. Review.
-
Survival of very young children with medulloblastoma (primitive neuroectodermal tumor of the posterior fossa) treated with craniospinal irradiation.Int J Radiat Oncol Biol Phys. 1998 Dec 1;42(5):959-67. doi: 10.1016/s0360-3016(98)00262-4. Int J Radiat Oncol Biol Phys. 1998. PMID: 9869216 Review.
References
-
- Childhood Cancer Statistics. England Annual report 2018. UK: Public Health England.
-
- Oeffinger KC, Mertens AC, Sklar CA, et al.; Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. - PubMed
-
- MacDonald C, Theurer JA, Doyle PC.. “Cured” but not “healed”: The application of principles of palliative care to cancer survivorship. Soc Sci Med. 2021;275(1):113802. - PubMed
-
- Khalil J, Chaabi S, Oberlin O, et al.. Medulloblastoma in childhood: What effects on neurocognitive functions? Cancer Radiother. 2019;23(5):370–377. - PubMed
-
- Limond JA, Bull KS, Calaminus G, et al.; Brain Tumour Quality of Survival Group, International Society of Paediatric Oncology (Europe; SIOP-E). Quality of survival assessment in European childhood brain tumour trials, for children aged 5 years and over. Eur J Paediatr Neurol. 2015;19(2):202–210. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
