pH-Responsive Mesoporous Silica Nanoparticles Loaded with Naringin for Targeted Osteoclast Inhibition and Bone Regeneration
- PMID: 38946884
- PMCID: PMC11213539
- DOI: 10.2147/IJN.S456545
pH-Responsive Mesoporous Silica Nanoparticles Loaded with Naringin for Targeted Osteoclast Inhibition and Bone Regeneration
Abstract
Background: It is well-established that osteoclast activity is significantly influenced by fluctuations in intracellular pH. Consequently, a pH-sensitive gated nano-drug delivery system represents a promising therapeutic approach to mitigate osteoclast overactivity. Our prior research indicated that naringin, a natural flavonoid, effectively mitigates osteoclast activity. However, naringin showed low oral availability and short half-life, which hinders its clinical application. We developed a drug delivery system wherein chitosan, as gatekeepers, coats mesoporous silica nanoparticles loaded with naringin (CS@MSNs-Naringin). However, the inhibitory effects of CS@MSNs-Naringin on osteoclasts and the underlying mechanisms remain unclear, warranting further research.
Methods: First, we synthesized CS@MSNs-Naringin and conducted a comprehensive characterization. We also measured drug release rates in a pH gradient solution and verified its biosafety. Subsequently, we investigated the impact of CS@MSNs-Naringin on osteoclasts induced by bone marrow-derived macrophages, focusing on differentiation and bone resorption activity while exploring potential mechanisms. Finally, we established a rat model of bilateral critical-sized calvarial bone defects, in which CS@MSNs-Naringin was dispersed in GelMA hydrogel to achieve in situ drug delivery. We observed the ability of CS@MSNs-Naringin to promote bone regeneration and inhibit osteoclast activity in vivo.
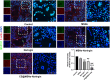
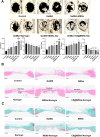
Results: CS@MSNs-Naringin exhibited high uniformity and dispersity, low cytotoxicity (concentration≤120 μg/mL), and significant pH sensitivity. In vitro, compared to Naringin and MSNs-Naringin, CS@MSNs-Naringin more effectively inhibited the formation and bone resorption activity of osteoclasts. This effect was accompanied by decreased phosphorylation of key factors in the NF-κB and MAPK signaling pathways, increased apoptosis levels, and a subsequent reduction in the production of osteoclast-specific genes and proteins. In vivo, CS@MSNs-Naringin outperformed Naringin and MSNs-Naringin, promoting new bone formation while inhibiting osteoclast activity to a greater extent.
Conclusion: Our research suggested that CS@MSNs-Naringin exhibited the strikingly ability to anti-osteoclasts in vitro and in vivo, moreover promoted bone regeneration in the calvarial bone defect.
Keywords: chitosan; mesoporous silica nanoparticles; naringin; osteoclast; pH-sensitive gated nano-drug delivery system.
© 2024 Gong et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

