This is a preprint.
Serum protein and imaging biomarkers after intermittent steroid treatment in muscular dystrophy
- PMID: 38947030
- PMCID: PMC11213068
- DOI: 10.1101/2024.06.14.24308858
Serum protein and imaging biomarkers after intermittent steroid treatment in muscular dystrophy
Update in
-
Serum protein and imaging biomarkers after intermittent steroid treatment in muscular dystrophy.Sci Rep. 2024 Nov 20;14(1):28745. doi: 10.1038/s41598-024-79024-8. Sci Rep. 2024. PMID: 39567576 Free PMC article.
Abstract
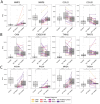
Background: Weekly Steroids in Muscular Dystrophy (WSiMD) was a pilot study to evaluate once weekly prednisone in patients with Limb Girdle and Becker muscular dystrophy (LGMD and BMD, respectively). At study endpoint, there were trends towards increased lean mass, reduced fat mass, reduced creatine kinase and improved motor function. The investigation was motivated by studies in mouse muscular dystrophy models in which once weekly glucocorticoid exposure enhanced muscle strength and reduced fibrosis.
Methods: WSiMD participants provided blood samples for aptamer serum profiling at baseline and after 6 months of weekly steroids. A subset completed magnetic resonance (MR) evaluation of muscle at study onset and endpoint.
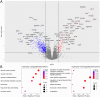
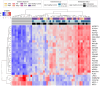
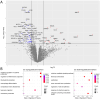
Results/conclusions: At baseline compared to age and sex-matched healthy controls, the aggregate serum protein profile in the WSiMD cohort was dominated by muscle proteins, reflecting leak of muscle proteins into serum. Disease status produced more proteins differentially present in serum compared to steroid-treatment effect. Nonetheless, a response to prednisone was discernable in the WSiMD cohort, even at this low dose. Glucocorticoids downregulated muscle proteins and upregulated certain immune process- and matrix-associated proteins. Muscle MR fat fraction showed trends with functional status. The prednisone-responsive markers could be used in larger trial of prednisone efficacy.
Keywords: MRI; glucocorticoid; limb girdle muscular dystrophy; muscle; muscular dystrophy; prednisone; serum biomarkers.
Conflict of interest statement
CONFLICTS Of INTEREST EMM has been a consultant to Amgen, AstraZeneca, Cytokinetics, Pfizer, Tenaya Therapeutics, and is a founder of Ikaika Therapeutics. ARD is CSO of Ikaika Therapeutics. The other authors have declared that no conflict of interest exists.
Figures
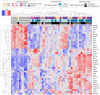

References
-
- Bushby K. et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Pharmacological and Psychosocial Management. Lancet Neurol 9, 77–93, (2010). - PubMed
-
- Fenichel G. M. et al. Long-Term Benefit from Prednisone Therapy in Duchenne Muscular Dystrophy. Neurology 41, 1874–1877, (1991). - PubMed
-
- Schara U., Mortier & Mortier W. Long-Term Steroid Therapy in Duchenne Muscular Dystrophy-Positive Results Versus Side Effects. J Clin Neuromuscul Dis 2, 179–183, (2001). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
