This is a preprint.
Head-to-head comparison of diagnostic accuracy of TB screening tests: Chest-X-ray, Xpert TB host response, and C-reactive protein
- PMID: 38947093
- PMCID: PMC11213098
- DOI: 10.1101/2024.06.20.24308402
Head-to-head comparison of diagnostic accuracy of TB screening tests: Chest-X-ray, Xpert TB host response, and C-reactive protein
Update in
-
Diagnostic accuracy of TB screening tests in a prospective multinational cohort: Chest-X-ray with computer-aided detection, Xpert TB host response, and C-reactive protein.Clin Infect Dis. 2024 Nov 7:ciae549. doi: 10.1093/cid/ciae549. Online ahead of print. Clin Infect Dis. 2024. PMID: 39509711
Abstract
Background: Accessible, accurate screening tests are necessary to advance tuberculosis (TB) case finding and early detection in high-burden countries. We compared the diagnostic accuracy of available TB triage tests.
Methods: We prospectively screened consecutive adults with ≥2 weeks of cough presenting to primary health centers in the Philippines, Vietnam, South Africa, Uganda, and India. All participants received the index tests: chest-X-ray (CXR), venous or capillary Cepheid Xpert TB Host Response (HR) testing, and point-of-care C-reactive protein (CRP) testing (Boditech iChroma II). CXR images were processed using computer-aided detection (CAD) algorithms. We assessed diagnostic accuracy against a microbiologic reference standard (sputum Xpert Ultra, culture). Optimal cut-points were chosen to achieve sensitivity ≥90% and maximize specificity. Two-test screening algorithms were considered, using two approaches: 1) sequential negative serial screening in which the second screening test is conducted only if the first is negative and positive is defined as positive on either test and 2) sequential positive serial screening, in which the second screening test is conducted only if the first is positive and positive is defined as positive on both tests.
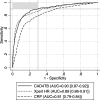
Results: Between July 2021 and August 2022, 1,392 participants with presumptive TB had valid results on index tests and the reference standard, and 303 (22%) had confirmed TB. In head-to-head comparisons, CAD4TB v7 showed the highest specificity when using a cut-point that achieves 90% sensitivity (70.3% vs. 65.1% for Xpert HR, difference 95% CI 1.6 to 8.9; 49.7% for CRP, difference 95% CI 17.0 to 24.3). Among the possible two-test screening algorithms, three met WHO target product profile (TPP) minimum accuracy thresholds and had higher accuracy than any test alone. At 90% sensitivity, the specificity was 79.6% for Xpert HR-CAD4TB [sequential negative], 75.9% for CRP-CAD4TB [sequential negative], and 73.7% for Xpert HR-CAD4TB [sequential positive].
Conclusions: CAD4TB achieves TPP targets and outperforms Xpert HR and CRP. Combining screening tests further increased accuracy. Cost and feasibility of two-test screening algorithms should be explored.
Registration: NCT04923958.
Figures

References
-
- World Health Organization. Global Tuberculosis Report. Geneva, Switzerland, 2023. 7 November 2023.
-
- WHO. High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting, 28–29 April 2014, Geneva, Switzerland. Geneva: World Health Organization, 2014. 2014.
-
- Kik SV, Gelaw SM, Ruhwald M, et al. Diagnostic accuracy of chest X-ray interpretation for tuberculosis by three artificial intelligence-based software in a screening use-case: an individual patient meta-analysis of global data. medRxiv 2022: 2022.01. 24.22269730.
-
- Gelaw SM, Kik SV, Ruhwald M, et al. Diagnostic accuracy of three computer-aided detection systems for detecting pulmonary tuberculosis on chest radiography when used for screening: Analysis of an international, multicenter migrants screening study. PLOS Glob Public Health 2023; 3(7): e0000402. - PMC - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
