Characterisation of sRNAs enriched in outer membrane vesicles of pathogenic Flavobacterium psychrophilum causing Bacterial Cold Water Disease in rainbow trout
- PMID: 38947174
- PMCID: PMC11212332
- DOI: 10.1002/jex2.161
Characterisation of sRNAs enriched in outer membrane vesicles of pathogenic Flavobacterium psychrophilum causing Bacterial Cold Water Disease in rainbow trout
Abstract
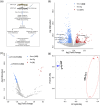
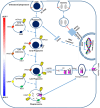
Flavobacterium psychrophilum (Fp) causes Bacterial Cold Water Disease in salmonids. During host-pathogen interactions, gram-negative bacteria, such as Fp, release external membrane vesicles (OMVs) harbouring cargos, such as DNA, RNA and virulence factors. This study aimed to characterise the potential role of the OMVs' small RNAs (sRNAs) in the Fp-rainbow trout host-pathogen interactions. sRNAs carried within OMVs were isolated from Fp. RNA-Seq datasets from whole-cell Fp and their isolated OMVs indicated substantial enrichment of specific sRNAs in the OMVs compared to the parent cell. Many of the OMV-packaged sRNAs were located in the pathogenicity islands of Fp. Conservation of sRNAs in 65 strains with variable degrees of virulence was reported. Dual RNA-Seq of host and pathogen transcriptomes on day 5 post-infection of Fp -resistant and -susceptible rainbow trout genetic lines revealed correlated expression of OMV-packaged sRNAs and their predicted host's immune gene targets. In vitro, treatment of the rainbow trout epithelial cell line RTgill-W1 with OMVs showed signs of cytotoxicity accompanied by dynamic changes in the expression of host genes when profiled 24 h following treatment. The OMV-treated cells, similar to the Fp -resistant fish, showed downregulated expression of the suppressor of cytokine signalling 1 (SOCS1) gene, suggesting induction of phagosomal maturation. Other signs of modulating the host gene expression following OMV-treatment include favouring elements from the phagocytic, endocytic and antigen presentation pathways in addition to HSP70, HSP90 and cochaperone proteins, which provide evidence for a potential role of OMVs in boosting the host immune response. In conclusion, the study identified novel microbial targets and inherent characteristics of OMVs that could open up new avenues of treatment and prevention of fish infections.
Keywords: OMVs; disease resistance; fish; small RNAs.
© 2024 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
LinkOut - more resources
Full Text Sources
