Severe thermal and major traumatic injury results in elevated plasma concentrations of total heme that are associated with poor clinical outcomes and systemic immune suppression
- PMID: 38947312
- PMCID: PMC11211257
- DOI: 10.3389/fimmu.2024.1416820
Severe thermal and major traumatic injury results in elevated plasma concentrations of total heme that are associated with poor clinical outcomes and systemic immune suppression
Abstract
Background: Traumatic and thermal injuries result in a state of systemic immune suppression, yet the mechanisms that underlie its development are poorly understood. Released from injured muscle and lysed red blood cells, heme is a damage associated molecular pattern with potent immune modulatory properties. Here, we measured plasma concentrations of total heme in over 200 traumatic and thermally-injured patients in order to examine its relationship with clinical outcomes and post-injury immune suppression.
Methods: Blood samples were collected from 98 burns (≥15% total body surface area) and 147 traumatically-injured (injury severity score ≥8) patients across the ultra-early (≤1 hour) and acute (4-72 hours) post-injury settings. Pro-inflammatory cytokine production by lipopolysaccharide (LPS) challenged whole blood leukocytes was studied, and plasma concentrations of total heme, and its scavengers haptoglobin, hemopexin and albumin measured, alongside the expression of heme-oxygenase-1 (HO-1) in peripheral blood mononuclear cells (PBMCs). LPS-induced tumour necrosis factor-alpha (TNF-α) production by THP-1 cells and monocytes following in vitro heme treatment was also examined.
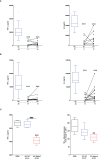
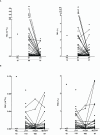
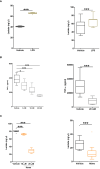
Results: Burns and traumatic injury resulted in significantly elevated plasma concentrations of heme, which coincided with reduced levels of hemopexin and albumin, and correlated positively with circulating levels of pro and anti-inflammatory cytokines. PBMCs isolated from trauma patients 4-12 and 48-72 hours post-injury exhibited increased HO-1 gene expression. Non-survivors of burn injury and patients who developed sepsis, presented on day 1 with significantly elevated heme levels, with a difference of 6.5 µM in heme concentrations corresponding to a relative 52% increase in the odds of post-burn mortality. On day 1 post-burn, heme levels were negatively associated with ex vivo LPS-induced TNF-α and interleukin-6 production by whole blood leukocytes. THP-1 cells and monocytes pre-treated with heme exhibited significantly reduced TNF-α production following LPS stimulation. This impairment was associated with decreased gene transcription, reduced activation of extracellular signal-regulated kinase 1/2 and an impaired glycolytic response.
Conclusions: Major injury results in elevated plasma concentrations of total heme that may contribute to the development of endotoxin tolerance and increase the risk of poor clinical outcomes. Restoration of the heme scavenging system could be a therapeutic approach by which to improve immune function post-injury.
Keywords: burns; critical care; heme; immune suppression; trauma.
Copyright © 2024 Tullie, Nicholson, Bishop, McGee, Asiri, Sullivan, Chen, Sardeli, Belli, Harrison, Moiemen, Lord and Hazeldine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

