New immune phenotypes for treatment response in high-grade serous ovarian carcinoma patients
- PMID: 38947323
- PMCID: PMC11211251
- DOI: 10.3389/fimmu.2024.1394497
New immune phenotypes for treatment response in high-grade serous ovarian carcinoma patients
Abstract
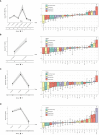
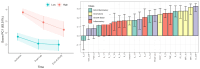
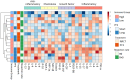
Despite advances in surgical and therapeutic approaches, high-grade serous ovarian carcinoma (HGSOC) prognosis remains poor. Surgery is an indispensable component of therapeutic protocols, as removal of all visible tumor lesions (cytoreduction) profoundly improves the overall survival. Enhanced predictive tools for assessing cytoreduction are essential to optimize therapeutic precision. Patients' immune status broadly reflects the tumor cell biological behavior and the patient responses to disease and treatment. Serum cytokine profiling is a sensitive measure of immune adaption and deviation, yet its integration into treatment paradigms is underexplored. This study is part of the IMPACT trial (NCT03378297) and aimed to characterize immune responses before and during primary treatment for HGSOC to identify biomarkers for treatment selection and prognosis. Longitudinal serum samples from 22 patients were collected from diagnosis until response evaluation. Patients underwent primary cytoreductive surgery or neoadjuvant chemotherapy (NACT) based on laparoscopy scoring. Twenty-seven serum cytokines analyzed by Bio-Plex 200, revealed two immune phenotypes at diagnosis: Immune High with marked higher serum cytokine levels than Immune Low. The immune phenotypes reflected the laparoscopy scoring and allocation to surgical treatment. The five Immune High patients undergoing primary cytoreductive surgery exhibited immune mobilization and extended progression-free survival, compared to the Immune Low patients undergoing the same treatment. Both laparoscopy and cytoreductive surgery induced substantial and transient changes in serum cytokines, with upregulation of the inflammatory cytokine IL-6 and downregulation of the multifunctional cytokines IP-10, Eotaxin, IL-4, and IL-7. Over the study period, cytokine levels uniformly decreased in all patients, leading to the elimination of the initial immune phenotypes regardless of treatment choice. This study reveals distinct pre-treatment immune phenotypes in HGSOC patients that might be informative for treatment stratification and prognosis. This potential novel biomarker holds promise as a foundation for improved assessment of treatment responses in patients with HGSOC. ClinicalTrials.gov Identifier: NCT03378297.
Keywords: HGSOC; RM-ASCA; cytokines; inflammation; longitudinal; ovarian cancer; surgery.
Copyright © 2024 Torkildsen, Austdal, Jarmund, Kleinmanns, Lamark, Nilsen, Stefansson, Sande, Iversen, Thomsen and Bjørge.
Conflict of interest statement
CT reports personal fees from AstraZeneca, Pfizer, and GlaxoSmithKline. LT reports personal fees from Bayer, Eisai Co. and AstraZeneca. LT and LB report financial support from AstraZeneca for this researcher-initiated trial. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. (2009) 115:1234–44. doi: 10.1002/cncr.24149 - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

