GSK2334470 attenuates high salt-exacerbated rheumatoid arthritis progression by restoring Th17/Treg homeostasis
- PMID: 38947509
- PMCID: PMC11214488
- DOI: 10.1016/j.isci.2024.109798
GSK2334470 attenuates high salt-exacerbated rheumatoid arthritis progression by restoring Th17/Treg homeostasis
Abstract
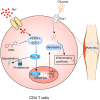
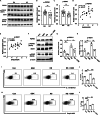
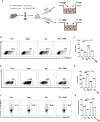
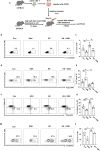
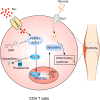
High salt (HS) consumption is a risk factor for multiple autoimmune disorders via disturbing immune homeostasis. Nevertheless, the exact mechanisms by which HS exacerbates rheumatoid arthritis (RA) pathogenesis remain poorly defined. Herein, we found that heightened phosphorylation of PDPK1 and SGK1 upon HS exposure attenuated FoxO1 expression to enhance the glycolytic capacity of CD4 T cells, resulting in strengthened Th17 but compromised Treg program. GSK2334470 (GSK), a dual PDPK1/SGK1 inhibitor, effectively mitigated the HS-induced enhancement in glycolytic capacity and the overproduction of IL-17A. Therefore, administration of GSK markedly alleviated HS-exacerbated RA progression in collagen-induced arthritis (CIA) model. Collectively, our data indicate that HS consumption subverts Th17/Treg homeostasis through the PDPK1-SGK1-FoxO1 signaling, while GSK could be a viable drug against RA progression in clinical settings.
Keywords: Biological sciences; Immunology; Natural sciences; Pharmacology; Physiology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Carranza-León D.A., Oeser A., Marton A., Wang P., Gore J.C., Titze J., Stein C.M., Chung C.P., Ormseth M.J. Tissue sodium content in patients with systemic lupus erythematosus: association with disease activity and markers of inflammation. Lupus. 2020;29:455–462. doi: 10.1177/0961203320908934. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

