Secretion of WNT7A by UC-MSCs assist in promoting the endometrial epithelial regeneration
- PMID: 38947517
- PMCID: PMC11214297
- DOI: 10.1016/j.isci.2024.109888
Secretion of WNT7A by UC-MSCs assist in promoting the endometrial epithelial regeneration
Abstract
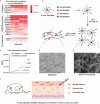
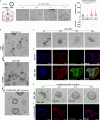
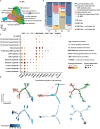
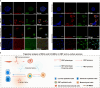
Stem cell therapy for intrauterine adhesions (IUAs) has been widely used in clinical treatment. However, intravenous injection lacks sufficient targeting capabilities, while in situ injection poses challenges in ensuring the effective survival of stem cells. Furthermore, the mechanism underlying the interaction between stem cells and endometrial cells in vivo remains poorly understood, and there is a lack of suitable in vitro models for studying these problems. Here, we designed an extracellular matrix (ECM)-adhesion mimic hydrogel for intrauterine administration, which was more effective than direct injection in treating IUAs. Additionally, we analyzed the epithelial-mesenchymal transition (EMT) and confirmed that the activation of endometrial epithelial stem cells is pivotal. Our findings demonstrated that umbilical cord mesenchymal stem cells (UC-MSCs) secrete WNT7A to activate endometrial epithelial stem cells, thereby accelerating regeneration of the endometrial epithelium. Concurrently, under transforming growth factor alpha (TGFA) stimulation secreted by the EMT epithelium, UC-MSCs upregulate E-cadherin while partially implanting into the endometrial epithelium.
Keywords: Cell biology; Female reproductive endocrinology; Stem cells research.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility.Acta Biomater. 2019 Jul 1;92:160-171. doi: 10.1016/j.actbio.2019.05.012. Epub 2019 May 7. Acta Biomater. 2019. PMID: 31075515
-
Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats.Sci Rep. 2022 Jan 10;12(1):412. doi: 10.1038/s41598-021-04454-7. Sci Rep. 2022. PMID: 35013490 Free PMC article.
-
Minimally invasive delivery of human umbilical cord-derived mesenchymal stem cells by an injectable hydrogel via Diels-Alder click reaction for the treatment of intrauterine adhesions.Acta Biomater. 2024 Mar 15;177:77-90. doi: 10.1016/j.actbio.2024.02.001. Epub 2024 Feb 6. Acta Biomater. 2024. PMID: 38331133
-
Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration.Mater Today Bio. 2021 Feb 25;11:100101. doi: 10.1016/j.mtbio.2021.100101. eCollection 2021 Jun. Mater Today Bio. 2021. PMID: 34036261 Free PMC article. Review.
-
A Promising Tool in Retina Regeneration: Current Perspectives and Challenges When Using Mesenchymal Progenitor Stem Cells in Veterinary and Human Ophthalmological Applications.Stem Cell Rev Rep. 2017 Oct;13(5):598-602. doi: 10.1007/s12015-017-9750-4. Stem Cell Rev Rep. 2017. PMID: 28643176 Free PMC article. Review.
Cited by
-
The Application of Hydrogels in the Treatment of Intrauterine Adhesions.Curr Pharm Des. 2025;31(13):1057-1066. doi: 10.2174/0113816128348746241030110806. Curr Pharm Des. 2025. PMID: 39754766 Review.
References
-
- Su J., Ding L., Cheng J., Yang J., Li X., Yan G., Sun H., Dai J., Hu Y. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum. Reprod. 2016;31:1075–1086. doi: 10.1093/humrep/dew041. - DOI - PubMed
LinkOut - more resources
Full Text Sources

