Neoadjuvant Immunotherapy Effectiveness in Patients With Microsatellite Instability-High (MSI-H) Gastric Cancer
- PMID: 38947586
- PMCID: PMC11214122
- DOI: 10.7759/cureus.61344
Neoadjuvant Immunotherapy Effectiveness in Patients With Microsatellite Instability-High (MSI-H) Gastric Cancer
Abstract
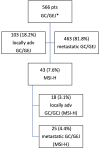
Purpose This research work evaluates monotherapy with checkpoint inhibitors (CPI). as a neoadjuvant treatment for patients with Microsatellite Instability-High (MSI-H) locally advanced gastric cancer. Methods Here we present the results of the retrospective study from Napalkov Cancer Center over 4.5 years on patients with MSI-H locally advanced gastric cancer. A total of 566 patients were analyzed, 18 of whom were included in the research, focusing on clinical response rate, surgical pathology, 'watch and wait' strategy, and safety outcomes on an exploratory basis. Patients were assigned to four to eight neoadjuvant cycles of CPI, followed by surgery. Results The objective response to neoadjuvant CPI in patients with MSI-H gastric cancer was 77.8%. Complete response was achieved in five (27.8%) and partial response in nine (50%) patients, accordingly. Surgery was performed on 14 patients. Complete margin-free (R0) resection rates were 100%. Downstaging was observed in 12 out of 14 patients. Histopathologic complete response rates (pathologic complete response or Tumor Regression Grade-major response (TRG1)) were achieved in eight (57.1%) patients. No disease progression was detected with a median follow-up of 33.7 months (4.4-55.7 months). Clinically significant adverse events were not observed. Conclusion CPI in a neoadjuvant setting for patients with MSI-H locally advanced gastric cancer is highly effective and safe.
Keywords: gastric cancer; immunotherapy; monotherapy; msi-h; neoadjuvant.
Copyright © 2024, Chubenko et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Cancer statistics, 2023. Siegel RL, Miller KD, Wagle NS, Jemal A. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 Trial. Lorenzen S, Götze TO, Thuss-Patience P, et al. J Clin Oncol. 2024;42:410–420. - PubMed
LinkOut - more resources
Full Text Sources
