An injectable magnesium-loaded hydrogel releases hydrogen to promote osteoporotic bone repair via ROS scavenging and immunomodulation
- PMID: 38948054
- PMCID: PMC11209720
- DOI: 10.7150/thno.97412
An injectable magnesium-loaded hydrogel releases hydrogen to promote osteoporotic bone repair via ROS scavenging and immunomodulation
Abstract
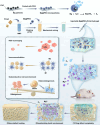
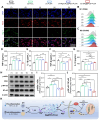
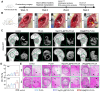
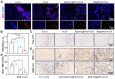
Background: The repair of osteoporotic bone defects remains challenging due to excessive reactive oxygen species (ROS), persistent inflammation, and an imbalance between osteogenesis and osteoclastogenesis. Methods: Here, an injectable H2-releasing hydrogel (magnesium@polyethylene glycol-poly(lactic-co-glycolic acid), Mg@PEG-PLGA) was developed to remodel the challenging bone environment and accelerate the repair of osteoporotic bone defects. Results: This Mg@PEG-PLGA gel shows excellent injectability, shape adaptability, and phase-transition ability, can fill irregular bone defect areas via minimally invasive injection, and can transform into a porous scaffold in situ to provide mechanical support. With the appropriate release of H2 and magnesium ions, the 2Mg@PEG-PLGA gel (loaded with 2 mg of Mg) displayed significant immunomodulatory effects through reducing intracellular ROS, guiding macrophage polarization toward the M2 phenotype, and inhibiting the IκB/NF-κB signaling pathway. Moreover, in vitro experiments showed that the 2Mg@PEG-PLGA gel inhibited osteoclastogenesis while promoting osteogenesis. Most notably, in animal experiments, the 2Mg@PEG-PLGA gel significantly promoted the repair of osteoporotic bone defects in vivo by scavenging ROS and inhibiting inflammation and osteoclastogenesis. Conclusions: Overall, our study provides critical insight into the design and development of H2-releasing magnesium-based hydrogels as potential implants for repairing osteoporotic bone defects.
Keywords: ROS scavenging; hydrogen; immunomodulation; magnesium-based hydrogel; osteoporotic bone defect.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

