Modularized supramolecular assemblies for hypoxia-activatable fluorescent visualization and image-guided theranostics
- PMID: 38948059
- PMCID: PMC11209709
- DOI: 10.7150/thno.95590
Modularized supramolecular assemblies for hypoxia-activatable fluorescent visualization and image-guided theranostics
Abstract
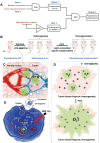
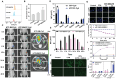
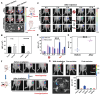
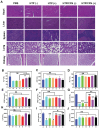
Rationale: Molecular imaging of microenvironment by hypoxia-activatable fluorescence probes has emerged as an attractive approach to tumor diagnosis and image-guided treatment. Difficulties remain in its translational applications due to hypoxia heterogeneity in tumor microenvironments, making it challenging to image hypoxia as a reliable proxy of tumor distribution. Methods: We report a modularized theranostics platform to fluorescently visualize hypoxia via light-modulated signal compensation to overcome tumor heterogeneity, thereby serving as a diagnostic tool for image-guided surgical resection and photodynamic therapy. Specifically, the platform integrating dual modules of fluorescence indicator and photodynamic moderator using supramolecular host-guest self-assembly, which operates cooperatively as a cascaded "AND" logic gate. First, tumor enrichment and specific fluorescence turn-on in hypoxic regions were accessible via tumor receptors and cascaded microenvironment signals as simultaneous inputs of the "AND" gate. Second, image guidance by a lighted fluorescence module and light-mediated endogenous oxygen consumption of a photodynamic module as dual inputs of "AND" gate collaboratively enabled light-modulated signal compensation in situ, indicating homogeneity of enhanced hypoxia-related fluorescence signals throughout a tumor. Results: In in vitro and in vivo analyses, the biocompatible platform demonstrated several strengths including a capacity for dual tumor targeting to progressively facilitate specific fluorescence turn-on, selective signal compensation, imaging-time window extension conducive to precise normalized image-guided treatment, and the functionality of tumor glutathione depletion to improve photodynamic efficacy. Conclusion: The hypoxia-activatable, image-guided theranostic platform demonstrated excellent potential for overcoming hypoxia heterogeneity in tumors.
Keywords: Tumor heterogeneity; fluorescent probe; hypoxia; photodynamic therapy; supramolecular assembly.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Pe'er D, Ogawa S, Elhanani O, Keren L, Oliver TG, Wedge D. Tumor heterogeneity. Cancer cell. 2021;39:1015–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

