Exploring the Aβ1-42 fibrillogenesis timeline by atomic force microscopy and surface enhanced Raman spectroscopy
- PMID: 38948077
- PMCID: PMC11211275
- DOI: 10.3389/fmolb.2024.1376411
Exploring the Aβ1-42 fibrillogenesis timeline by atomic force microscopy and surface enhanced Raman spectroscopy
Abstract
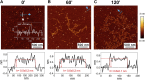
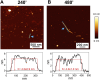
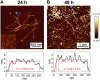
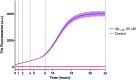
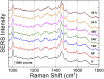
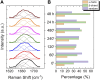
Introduction: Alzheimer's disease (AD) is a progressive debilitating neurological disorder representing the most common neurodegenerative disease worldwide. Although the exact pathogenic mechanisms of AD remain unresolved, the presence of extracellular amyloid-β peptide 1-42 (Aβ1-42) plaques in the parenchymal and cortical brain is considered one of the hallmarks of the disease. Methods: In this work, we investigated the Aβ1-42 fibrillogenesis timeline up to 48 h of incubation, providing morphological and chemo-structural characterization of the main assemblies formed during the aggregation process of Aβ1-42, by atomic force microscopy (AFM) and surface enhanced Raman spectroscopy (SERS), respectively. Results: AFM topography evidenced the presence of characteristic protofibrils at early-stages of aggregation, which form peculiar macromolecular networks over time. SERS allowed to track the progressive variation in the secondary structure of the aggregation species involved in the fibrillogenesis and to determine when the β-sheet starts to prevail over the random coil conformation in the aggregation process. Discussion: Our research highlights the significance of investigating the early phases of fibrillogenesis to better understand the molecular pathophysiology of AD and identify potential therapeutic targets that may prevent or slow down the aggregation process.
Keywords: AFM; Alzheimer’s disease; SERS; amyloid-β peptide; fibrillogenesis; neurodegeneration.
Copyright © 2024 Polykretis, D’Andrea, Banchelli, Napolitano, Cascella, de Angelis and Matteini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adamcik J., Mezzenga R. (2012). Study of amyloid fibrils via atomic force microscopy. Curr. Opin. Colloid and Interface Sci. 17, 369–376. 10.1016/j.cocis.2012.08.001 - DOI
-
- Alzheimer’s Association (2023). Annual report. Alzheimer’s disease and dementia. Available at: https://alz.org/about/annual-report (Accessed January 11, 2024).
-
- Banchelli M., Amicucci C., Ruggiero E., D’Andrea C., Cottat M., Ciofini D., et al. (2019). Spot-on SERS detection of biomolecules with laser-patterned dot arrays of assembled silver nanowires. ChemNanoMat 5, 1036–1043. 10.1002/cnma.201900035 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

