Nicotine flux and pharmacokinetics-based considerations for early assessment of nicotine delivery systems
- PMID: 38948427
- PMCID: PMC11214420
- DOI: 10.1016/j.dadr.2024.100245
Nicotine flux and pharmacokinetics-based considerations for early assessment of nicotine delivery systems
Abstract
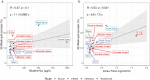
In the past few years, technological advancements enabled the development of novel electronic nicotine delivery systems (ENDS). Several empirical measures such as "nicotine flux" are being proposed to evaluate the abuse liability potential of these products. We explored the applicability of nicotine flux for clinical nicotine pharmacokinetics (PK) and 52-week quit success from cigarettes for a wide range of existing nicotine delivery systems. We found that the differences in nicotine flux for various nicotine delivery systems are not related to changes in PK, as nicotine flux does not capture key physiological properties such as nicotine absorption rate. Further, the 52-week quit success and abuse liability potential of nicotine nasal sprays (high nicotine flux product), and nicotine inhalers (nicotine flux similar to ENDS) are low, suggesting that nicotine flux is a poor metric for the assessment of nicotine delivery systems. PK indices are more dependable for characterizing nicotine delivery systems, and a nicotine plasma > 1 could improve 52-week quit success from cigarettes. However, a single metric may be inadequate to fully assess the abuse liability potential of nicotine delivery systems and needs to be further studied. A combination of in vitro and in silico approaches could potentially address the factors influencing the inhaled aerosol dosimetry and resulting PK of nicotine to provide early insights for ENDS assessments. Further research is required to understand nicotine dosimetry and PK for ad libitum product use, and abuse liability indicators of nicotine delivery systems. This commentary is intended to (1) highlight the need to think beyond a single empirical metric such as nicotine flux, (2) suggest potential PK-based metrics, (3) suggest the use of in vitro and in silico tools to obtain early insights into inhaled aerosol dosimetry for ENDS, and (4) emphasize the importance of considering comprehensive clinical pharmacology outcomes to evaluate nicotine delivery systems.
Keywords: Electronic nicotine delivery systems; Nicotine dose; Nicotine flux; Nicotine pharmacokinetics; abuse liability.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Aditya R. Kolli reports financial support was provided by Philip Morris International. Jed E. Rose reports a relationship with Philip Morris International that includes: funding grants. Aditya R. Kolli, Emilija Veljkovic, Florian Calvino-Martin, Arkadiusz K. Kuczaj, Marco Esposito, and Ondrej Koumalare employees of Philip Morris International. Manuel C. Peitsch was an employee of Philip Morris International when this work was performed. Jed E. Rose discloses research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, Embera Neurotherapeutics, Inc., Otsuka Pharmaceutical, and JUUL Labs; consulting with Revive Pharmaceuticals; and consulting and patent purchase agreements with Philip Morris International.
Figures



References
-
- Allain F., Minogianis E.A., Roberts D.C., Samaha A.N. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci. Biobehav. Rev. 2015;56:166–179. - PubMed
-
- Anjilvel S., Asgharian B. A multiple-path model of particle deposition in the rat lung. Fundam. Appl. Toxicol. 1995;28:41–50. - PubMed
-
- Asgari M., Lucci F., Bialek J., Dunan B., Andreatta G., Smajda R., Lani S., Blondiaux N., Majeed S., Steiner S., Schaller J.-P., Frentzel S., Hoeng J., Kuczaj A.K. Development of a realistic human respiratory tract cast representing physiological thermal conditions. Aerosol Sci. Technol. 2019:1–33.
-
- Asgharian B., Price O., Creel A., Chesnutt J., Schroeter J., Fallica J., Erives G., Rasheed N., Chemerynski S. Simulation modeling of air and droplet temperatures in the human respiratory tract for inhaled tobacco products. Ann. Biomed. Eng. 2022 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

