Deciphering Natural Killer Cell Cytotoxicity Against Medulloblastoma in vitro and in vivo: Implications for Immunotherapy
- PMID: 38948503
- PMCID: PMC11214763
- DOI: 10.2147/ITT.S458278
Deciphering Natural Killer Cell Cytotoxicity Against Medulloblastoma in vitro and in vivo: Implications for Immunotherapy
Abstract
Purpose: Medulloblastoma (MB) is the most prevalent paediatric brain tumour. Despite improvements in patient survival with current treatment strategies, the quality of life of these patients remains poor owing to the sequelae and relapse risk. An alternative, or, in addition to the current standard treatment, could be considered immunotherapy, such as Natural Killer cells (NK). NK cells are cytotoxic innate lymphoid cells that play a major role in cancer immunosurveillance. To date, the mechanism of cytotoxicity of NK cells, especially regarding the steps of adhesion, conjugation, cytotoxic granule polarisation in the cell contact area, perforin and granzyme release in two and three dimensions, and therapeutic efficacy in vivo have not been precisely described.
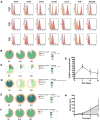
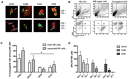
Materials and methods: Each step of NK cytotoxicity against the three MB cell lines was explored using confocal microscopy for conjugation, Elispot for degranulation, flow cytometry, and luminescence assays for target cell necrosis and lysis and mediators released by cytokine array, and then confirmed in a 3D spheroid model. Medulloblastoma-xenografted mice were treated with NK cells. Their persistence was evaluated by flow cytometry, and their efficacy in tumour growth and survival was determined. In addition, their effects on the tumour transcriptome were evaluated.
Results: NK cells showed variable affinities for conjugation with MB target cells depending on their subgroup and cytokine activation. Chemokines secreted during NK and MB cell co-culture are mainly associated with angiogenesis and immune cell recruitment. NK cell cytotoxicity induces MB cell death in both 2D and 3D co-culture models. NK cells initiated an inflammatory response in a human MB murine model by modulating the MB cell transcriptome.
Conclusion: Our study confirmed that NK cells possess both in vitro and in vivo cytotoxic activity against MB cells and are of interest for the development of immunotherapy.
Keywords: adoptive transfer; cancer; immune cells; medulloblastoma.
© 2024 Gauthier et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
-
- Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21(8):1581–1591. doi:10.1200/JCO.2003.05.116 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

