Evaluation of bone marrow glucose uptake and adiposity in male rats after diet and exercise interventions
- PMID: 38948514
- PMCID: PMC11211282
- DOI: 10.3389/fendo.2024.1422869
Evaluation of bone marrow glucose uptake and adiposity in male rats after diet and exercise interventions
Abstract
Objectives: Obesity impairs bone marrow (BM) glucose metabolism. Adult BM constitutes mostly of adipocytes that respond to changes in energy metabolism by modulating their morphology and number. Here we evaluated whether diet or exercise intervention could improve the high-fat diet (HFD) associated impairment in BM glucose uptake (BMGU) and whether this associates with the morphology of BM adipocytes (BMAds) in rats.
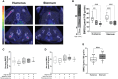
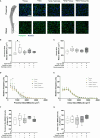
Methods: Eight-week-old male Sprague-Dawley rats were fed ad libitum either HFD or chow diet for 24 weeks. Additionally after 12 weeks, HFD-fed rats switched either to chow diet, voluntary intermittent running exercise, or both for another 12 weeks. BMAd morphology was assessed by perilipin-1 immunofluorescence staining in formalin-fixed paraffin-embedded tibial sections. Insulin-stimulated sternal and humeral BMGU were measured using [18F]FDG-PET/CT. Tibial microarchitecture and mineral density were measured with microCT.
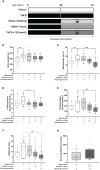
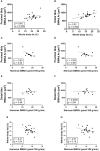
Results: HFD rats had significantly higher whole-body fat percentage compared to the chow group (17% vs 13%, respectively; p = 0.004) and larger median size of BMAds in the proximal tibia (815 µm2 vs 592 µm2, respectively; p = 0.03) but not in the distal tibia. Switch to chow diet combined with running exercise normalized whole-body fat percentage (p < 0.001) but not the BMAd size. At 32 weeks of age, there was no significant difference in insulin-stimulated BMGU between the study groups. However, BMGU was significantly higher in sternum compared to humerus (p < 0.001) and higher in 8-week-old compared to 32-week-old rats (p < 0.001). BMAd size in proximal tibia correlated positively with whole-body fat percentage (r = 0.48, p = 0.005) and negatively with humeral BMGU (r = -0.63, p = 0.02). HFD significantly reduced trabecular number (p < 0.001) compared to the chow group. Switch to chow diet reversed this as the trabecular number was significantly higher (p = 0.008) than in the HFD group.
Conclusion: In this study we showed that insulin-stimulated BMGU is age- and site-dependent. BMGU was not affected by the study interventions. HFD increased whole-body fat percentage and the size of BMAds in proximal tibia. Switching from HFD to a chow diet and running exercise improved glucose homeostasis and normalized the HFD-induced increase in body fat but not the hypertrophy of BMAds.
Keywords: bone marrow adiposity; bone marrow glucose uptake; diet; exercise; positron emission tomography.
Copyright © 2024 Ojala, Widjaja, Hentilä, Jalo, Helin, Nissinen, Jalava, Eskola, Rajander, Löyttyniemi, Ivaska and Hannukainen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

