This is a preprint.
Liver-specific Mettl14 deletion induces nuclear heterotypia and dysregulates RNA export machinery
- PMID: 38948765
- PMCID: PMC11212911
- DOI: 10.1101/2024.06.17.599413
Liver-specific Mettl14 deletion induces nuclear heterotypia and dysregulates RNA export machinery
Abstract
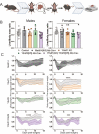
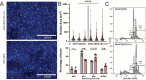
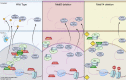
Modification of RNA with N6-methyladenosine (m6A) has gained attention in recent years as a general mechanism of gene regulation. In the liver, m6A, along with its associated machinery, has been studied as a potential biomarker of disease and cancer, with impacts on metabolism, cell cycle regulation, and pro-cancer state signaling. However these observational data have yet to be causally examined in vivo. For example, neither perturbation of the key m6A writers Mettl3 and Mettl14, nor the m6A readers Ythdf1 and Ythdf2 have been thoroughly mechanistically characterized in vivo as they have been in vitro. To understand the functions of these machineries, we developed mouse models and found that deleting Mettl14 led to progressive liver injury characterized by nuclear heterotypia, with changes in mRNA splicing, processing and export leading to increases in mRNA surveillance and recycling.
Conflict of interest statement
Competing interests All authors declare they have no competing interests.
Figures





References
-
- Berggren K. A., Schwartz R. E., Kleiner R. E., Ploss A., The impact of epitranscriptomic modifications on liver disease. Trends Endocrinol Metab 35 (2024). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
