This is a preprint.
Top-Down Proteomics Identifies Plasma Proteoform Signatures of Liver Cirrhosis Progression
- PMID: 38948836
- PMCID: PMC11212939
- DOI: 10.1101/2024.06.19.599662
Top-Down Proteomics Identifies Plasma Proteoform Signatures of Liver Cirrhosis Progression
Update in
-
Top-Down Proteomics Identifies Plasma Proteoform Signatures of Liver Cirrhosis Progression.Mol Cell Proteomics. 2024 Dec;23(12):100876. doi: 10.1016/j.mcpro.2024.100876. Epub 2024 Nov 8. Mol Cell Proteomics. 2024. PMID: 39521382 Free PMC article.
Abstract
Cirrhosis, advanced liver disease, affects 2-5 million Americans. While most patients have compensated cirrhosis and may be fairly asymptomatic, many decompensate and experience life-threatening complications such as gastrointestinal bleeding, confusion (hepatic encephalopathy), and ascites, reducing life expectancy from 12 to less than 2 years. Among patients with compensated cirrhosis, identifying patients at high risk of decompensation is critical to optimize care and reduce morbidity and mortality. Therefore, it is important to preferentially direct them towards specialty care which cannot be provided to all patients with cirrhosis. We used discovery Top-down Proteomics (TDP) to identify differentially expressed proteoforms (DEPs) in the plasma of patients with progressive stages of liver cirrhosis with the ultimate goal to identify candidate biomarkers of disease progression. In this pilot study, we identified 209 DEPs across three stages of cirrhosis (compensated, compensated with portal hypertension, and decompensated), of which 115 derived from proteins enriched in the liver at a transcriptional level and discriminated the three stages of cirrhosis. Enrichment analyses demonstrated DEPs are involved in several metabolic and immunological processes known to be impacted by cirrhosis progression. We have preliminarily defined the plasma proteoform signatures of cirrhosis patients, setting the stage for ongoing discovery and validation of biomarkers for early diagnosis, risk stratification, and disease monitoring.
Conflict of interest statement
Conflict-of-interest statement: NLK is involved in entrepreneurial activities in top-down proteomics and consults for Thermo Fisher Scientific. RDM is a current Thermo Fisher Scientific employee. The other authors have declared that no conflict of interest exists.
Figures
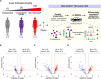


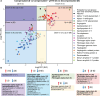

References
-
- Xu J.; Murphy S. L.; Kochanek K. D.; Bastian B.; Arias E. Deaths: Final Data for 2016. Natl Vital Stat Rep 2018, 67 (5), 1–76. - PubMed
-
- Collaborators G. B. D. C. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020, 5 (3), 245–266. DOI: 10.1016/S2468-1253(19)30349-8. - DOI - PMC - PubMed
-
- Ladner D. P.; Gmeiner M.; Hasjim B. J.; Mazumder N.; Kang R.; Parker E.; Stephen J.; Polineni P.; Chorniy A.; Zhao L.; et al. Increasing prevalence of cirrhosis among insured adults in the United States, 2012–2018. PLoS One 2024, 19 (2), e0298887. DOI: 10.1371/journal.pone.0298887. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
