Efficacy and safety of bendamustine-containing bridging therapy in R/R LBCL patients receiving CD19 CAR T-cells
- PMID: 38948924
- PMCID: PMC11208722
- DOI: 10.1002/hem3.86
Efficacy and safety of bendamustine-containing bridging therapy in R/R LBCL patients receiving CD19 CAR T-cells
Abstract
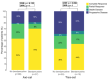
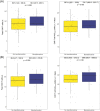
Bridging therapy (BT) after leukapheresis is required in most relapsed/refractory (R/R) large B-cell lymphoma (LBCL) patients receiving chimeric antigen receptor (CAR) T cells. Bendamustine-containing regimens are a potential BT option. We aimed to assess if this agent had a negative impact on CAR-T outcomes when it was administered as BT. We included R/R LBCL patients from six centers who received systemic BT after leukapheresis from February 2019 to September 2022; patients who only received steroids or had pre-apheresis bendamustine exposure were excluded. Patients were divided into two BT groups, with and without bendamustine. Separate safety and efficacy analyses were carried out for axi-cel and tisa-cel. Of 243 patients who received BT, bendamustine (benda) was included in 62 (26%). There was a higher rate of BT progressors in the non-benda group (62% vs. 45%, p = 0.02). Concerning CAR-T efficacy, complete responses were comparable for benda versus non-benda BT cohorts with axi-cel (70% vs. 53%, p = 0.12) and tisa-cel (44% vs. 36%, p = 0.70). Also, 12-month progression-free and overall survival were not significantly different between BT groups with axi-cel (56% vs. 43% and 71% vs. 63%) and tisa-cel (25% vs. 26% and 52% vs. 48%); there were no differences when BT response was considered. CAR T-cell expansion for each construct was similar between BT groups. Regarding safety, CRS G ≥3 (6% vs. 6%, p = 0.79), ICANS G ≥3 (15% vs. 17%, p = 0.68), severe infections, and neutropenia post-infusion were comparable among BT regimens. BT with bendamustine-containing regimens is safe for patients requiring disease control during CAR T-cell manufacturing.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Gloria Iacoboni: Consultancy and Honoraria: Novartis, Roche, Kite/Gilead, Bristol‐Myers Squibb, Abbvie, Janssen, Sandoz, Miltenyi, AstraZeneca. Mario A. Sánchez‐Salinas Honoraria for presentations: Kite. Support for attending meetings: Takeda. Kai Rejeski: Kite/Gilead: Research Funding, Consultancy, Honoraria and travel support; Novartis: Honoraria; BMS/Celgene: Consultancy, Honoraria; Pierre‐Fabre: travel support. Mi Kwon Consulting and lectures: Gilead, Jazz, Pfizer. Katarzyna A. Jalowiec Honoraria: Kite/Gilead. Rafael Hernani: Research: Gilead. Travel support: Gilead. Honoraria: Gilead, Janssen, MSD, Celgene, Novartis. Viktoria Blumenberg: BMS/Celgene: Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Janssen: Research Funding, Honoraria; Novartis: Research Funding, Honoraria; Roche: Consultancy, Research Funding; Takeda: Research Funding. Claire Roddie: Honoraria from Kite/Gilead, Novartis, BMS, Amgen. Javier Delgado‐Serrano: Honoraria from Kite‐Gilead, Novartis, Bristol Myers Squibb, Janssen. Rebeca Bailén: Speaker and travel: Kite. Cecilia Carpio: Regeneron: Consultancy/Advisory, BMS: Consultancy/Advisory, Takeda: Consultancy/Advisory/Honoraria, Novartis: Honoraria. Marion Subklewe: receives industry research support from Amgen, Bristol‐Myers Squibb/Celgene, Gilead, Janssen, Miltenyi Biotec, Morphosys, Novartis, Roche, Seattle Genetics, and Takeda, and serves as a consultant/advisor to AvenCell, CDR‐Life, Ichnos Sciences, Incyte Biosciences, Janssen, Molecular Partners, and Takeda. She serves on the speakers' bureau at Amgen, AstraZeneca, BMS/Celgene, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, and Takeda. Maeve O'Reilly: Honoraria from Kite, Novartis, Janssen. Advisory boards Kite and Autolus. Travel grant Kite and Novartis. Pere Barba: Allogene: Honoraria; Amgen: Honoraria; BMS: Honoraria; Kite/Gilead: Honoraria; Janssen: Honoraria; Jazz Pharmaceuticals: Honoraria; Miltenyi: Honoraria; Novartis: Honoraria; Nektar: Honoraria.
Figures


References
-
- Schuster SJ, Tam CS, Borchmann P, et al. Long‐term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B‐cell lymphomas (JULIET): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2021;22(10):1403‐1415. 10.1016/S1470-2045(21)00375-2 - DOI - PubMed
