COVID-19 Vaccine Effectiveness in Autumn and Winter 2022 to 2023 Among Older Europeans
- PMID: 38949812
- PMCID: PMC11217869
- DOI: 10.1001/jamanetworkopen.2024.19258
COVID-19 Vaccine Effectiveness in Autumn and Winter 2022 to 2023 Among Older Europeans
Abstract
Importance: In the context of emerging SARS-CoV-2 variants or lineages and new vaccines, it is key to accurately monitor COVID-19 vaccine effectiveness (CVE) to inform vaccination campaigns.
Objective: To estimate the effectiveness of COVID-19 vaccines administered in autumn and winter 2022 to 2023 against symptomatic SARS-CoV-2 infection (with all circulating viruses and XBB lineage in particular) among people aged 60 years or older in Europe, and to compare different CVE approaches across the exposed and reference groups used.
Design, setting, and participants: This case-control study obtained data from VEBIS (Vaccine Effectiveness, Burden and Impact Studies), a multicenter study that collects COVID-19 and influenza data from 11 European sites: Croatia; France; Germany; Hungary; Ireland; Portugal; the Netherlands; Romania; Spain, national; Spain, Navarre region; and Sweden. Participants were primary care patients aged 60 years or older with acute respiratory infection symptoms who were recruited at the 11 sites after the start of the COVID-19 vaccination campaign from September 2022 to August 2023. Cases and controls were defined as patients with positive and negative, respectively, reverse transcription-polymerase chain reaction (RT-PCR) test results.
Exposures: The exposure was COVID-19 vaccination. The exposure group consisted of patients who received a COVID-19 vaccine during the autumn and winter 2022 to 2023 vaccination campaign and 14 days or more before symptom onset. Reference group included patients who were not vaccinated during or in the 6 months before the 2022 to 2023 campaign (seasonal CVE), those who were never vaccinated (absolute CVE), and those who were vaccinated with at least the primary series 6 months or more before the campaign (relative CVE). For relative CVE of second boosters, patients receiving their second booster during the campaign were compared with those receiving 1 booster 6 months or more before the campaign.
Main outcomes and measures: The outcome was RT-PCR-confirmed, medically attended, symptomatic SARS-CoV-2 infection. Four CVE estimates were generated: seasonal, absolute, relative, and relative of second boosters. CVE was estimated using logistic regression, adjusting for study site, symptom onset date, age, chronic condition, and sex.
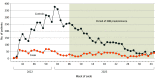
Results: A total of 9308 primary care patients were included, with 1687 cases (1035 females; median [IQR] age, 71 [65-79] years) and 7621 controls (4619 females [61%]; median [IQR] age, 71 [65-78] years). Within 14 to 89 days after vaccination, seasonal CVE was 29% (95% CI, 14%-42%), absolute CVE was 39% (95% CI, 6%-60%), relative CVE was 31% (95% CI, 15% to 44%), and relative CVE of second boosters was 34% (95% CI, 18%-47%) against all SARS-CoV-2 variants. In the same interval, seasonal CVE was 44% (95% CI, -10% to 75%), absolute CVE was 52% (95% CI, -23% to 82%), relative CVE was 47% (95% CI, -8% to 77%), and relative CVE of second boosters was 46% (95% CI, -13% to 77%) during a period of high XBB circulation. Estimates decreased with time since vaccination, with no protection from 180 days after vaccination.
Conclusions and relevance: In this case-control study among older Europeans, all CVE approaches suggested that COVID-19 vaccines administered in autumn and winter 2022 to 2023 offered at least 3 months of protection against symptomatic, medically attended, laboratory-confirmed SARS-CoV-2 infection. The effectiveness of new COVID-19 vaccines against emerging SARS-CoV-2 variants should be continually monitored using CVE seasonal approaches.
Conflict of interest statement
Figures

References
-
- European Centre for Disease Prevention and Control . Data on SARS-CoV-2 variants in the EU/EEA. 2023. Accessed December 4, 2023. https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covi...
-
- European Medicines Agency . First adapted COVID-19 booster vaccines recommended for approval in the EU. 2022. Accessed May 16, 2024. https://www.ema.europa.eu/en/news/first-adapted-covid-19-booster-vaccine...
-
- European Centre for Disease Prevention and Control . Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA. 2022. Accessed May 16, 2024. https://www.ecdc.europa.eu/sites/default/files/documents/Overview-vaccin...
-
- Haute Autorité de Santé . Covid-19: la HAS intègre les vaccins bivalents dans la stratégie de vaccination pour l’automne. 2022. Accessed May 16, 2024. https://www.has-sante.fr/jcms/p_3368002/fr/covid-19-la-has-integre-les-v...
-
- Link-Gelles R, Ciesla AA, Roper LE, et al. . Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults—increasing community access to testing program, United States, December 2022-January 2023. MMWR Morb Mortal Wkly Rep. 2023;72(5):119-124. doi:10.15585/mmwr.mm7205e1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

